Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help! Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important
please help! 
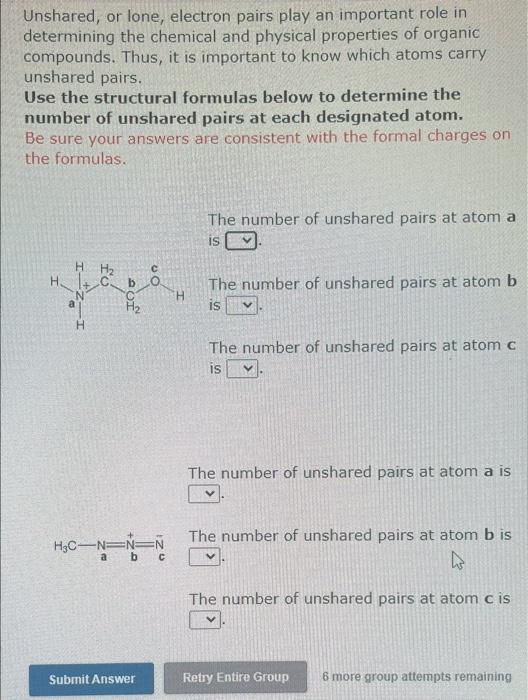
Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is 207 The number of unshared pairs at atom b is CH * CHE The number of unshared pairs at atom c is The number of unshared pairs at atom a is CO The number of unshared pairs at atom b is Hoc CH3 The number of unshared pairs at atom cis Submit Answer Retry Entire Group 6 more group attempts remaining Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is H H2 H On The number of unshared pairs at atom b AOT H IS H The number of unshared pairs at atom c IS The number of unshared pairs at atom a is The number of unshared pairs at atom b is H3C-N=N=N ab The number of unshared pairs at atom cis Submit Answer Retry Entire Group 6 more group attempts remaining 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


