Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with all. thank you The following initial rate data are for the oxidation of nitrogen monoxide by oxygen at 25C : 2NO+O22NO2 Complete
please help with all. thank you 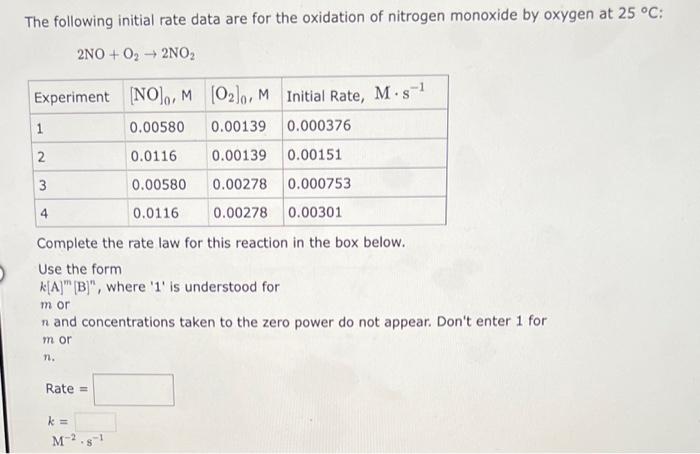

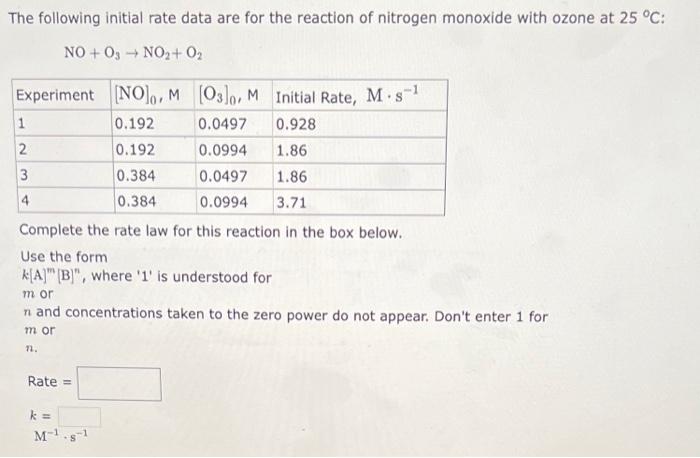
The following initial rate data are for the oxidation of nitrogen monoxide by oxygen at 25C : 2NO+O22NO2 Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n, where ' 1 ' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n. Rate = k= M2s1 The following initial rate data are for the reaction of hypochlorite ion with iodide ion in 1M aqueous hydroxide solution: OCl+IOI+Cl Complete the rate law for this reaction in the box below. Use the form k[A]m[B], where ' 1 ' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n. Rate = k= M1s1 The following initial rate data are for the reaction of nitrogen monoxide with ozone at 25C : NO+O3NO2+O2 Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n, where ' 1 ' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n. Rate = k= M1s1 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


