Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with all The freezing point of water H2O is 0.00C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in water is sucrose. How
please help with all 

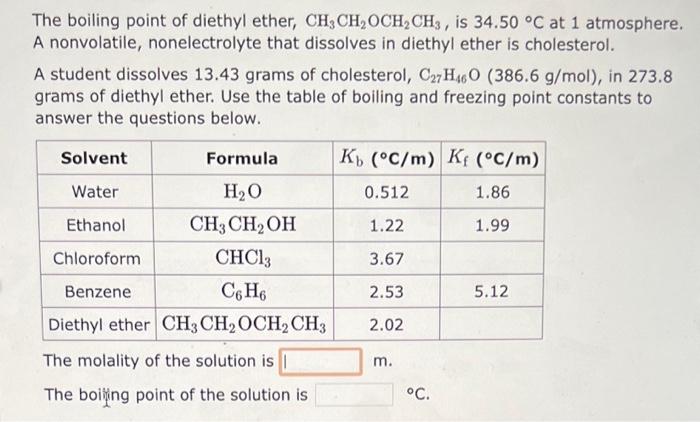
The freezing point of water H2O is 0.00C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in water is sucrose. How many grams of sucrose, C12H22O11(342.3g/mol), must be dissolved in 225.0 grams of water to reduce the freezing point by 0.400C ? Refer to the table for the necessary boiling or freezing point constant. The boiling point of water H2O is 100.0C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in water is urea. How many grams of urea, CH4N2O(60.10g/mol), must be dissolved in 242.0 grams of water to raise the boiling point by 0.300C ? Refer to the table for the necessary boiling or freezing point constant. The boiling point of diethyl ether, CH3CH2OCH2CH3, is 34.50C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in diethyl ether is cholesterol. A student dissolves 13.43 grams of cholesterol, C27H46O(386.6g/mol), in 273.8 grams of diethyl ether. Use the table of boiling and freezing point constants to answer the questions below. The molality of the solution is m. The boiling point of the solution is C 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


