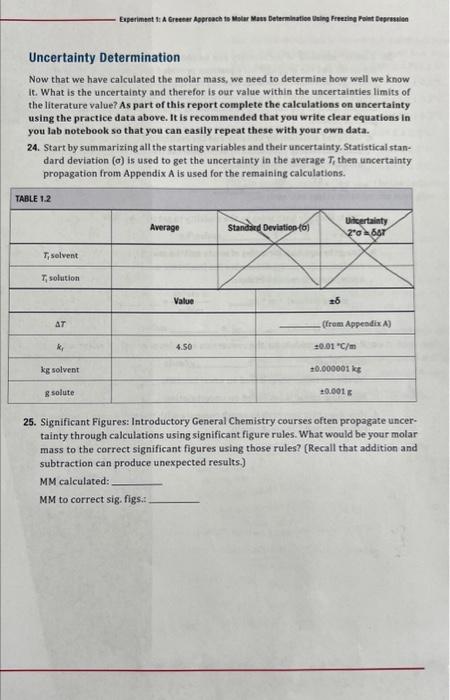
please help with number 24-26

Practice Calculations Use the following data to answer the questions below. Assume 9.045 g of stearic acid, and 1.327g of unknown for the mixture runs 4-6. Assume the k8 is 4.50Ckg/mole. Assume the uncertainty is 1 in the last digit ( 0.001g for the masses and 0.01 for k1 ]. Run 1: Solvent T/hutes=68.90.2C Run 2. Solvent Tfewent=69.00.2C Run 3: Solvent Tfotm=67.80.2C Run 4: Mixture 2gT2t=64.60.3C Run 5: Mixture 2gT2t=64.30.3C Run 6: Mixture 28T2t=64.70.3C 22. In groups, create a flow chart below including the equations required to process the raw data into molar mass for the runs given. Indicate when values are averaged and 7s are determined. - calcuate Ang Te for solvent - Tr ni mix (solute + jolvent) - determine acprision of freeting peim for each of 4,5,6 hins - we have, T=k6m malimy ving this determine, mplalit (tori/kg) - form mas of svivent inikg, estimate msles of selvite - for each von determine morar mase a majul molea - hiverge mese to Jet moviar mals 23. Using the data above, in your lab notebook set up a table that will clearly show the progression of data from raw data to final molar mass for the data above (see Table 1.2). Note: The masses for your experiment will be different as they will be 2g. Molality for 1.327 grams: .911 .024-evley Molar Mass for 1.327 grams: 161.2e4,2 TF=65.62AugJmike364.6+64.3+64.7=64.5nadetion157.0Tnax=64.5+,3Shltactimn165.3(6=kkxmn2157.0+105.3=161.2=16h2157.6=165316k.2=4.2m1=(4.9+.01)(6t.6+.2)(64.5+.3)=.177mov/ksmolarmasi146124.2(68.62)(64.53)=(4.5001)m2m2=(4.50.01)(6.6.2)((64.5.3)935mol/kgm=227+.n35=.911molkgd=AIl-.5f7.935.911=.024 Uncertainty Determination Now that we have calculated the molar mass, we need to determine how well we know It. What is the uncertainty and therefor is our value within the uncertainties limits of the literature value? As part of this report complete the calculations on uncertainty using the practice data above. It is recommended that you write clear equations in you lab notebook so that you can easily repeat these with your own data. 24. Start by summarizing all the starting variables and their uncertainty. Statistical standard deviation () is used to get the uncertainty in the average T, then uncertainty propagation from Appendix A is used for the remaining calculations. 25. Significant Figures: Introductory General Chemistry courses often propagate uncertainty through calculations using significant figure rules. What would be your molar mass to the correct significant figures using those rules? (Recall that addition and subtraction can produce unexpected results.) MM calculated: MM to correct sig. ftgs:t 26. A better estimate of uncertainty can be obtained by the combination of standard deviation and the propagation equations previded in Appendix A. We will fimit cur Investigation to the randem uncertainty calculations Because this aext equation is all multiplication and division, this can be done in one cquation wing all of the rariabies that are used in the complete calculation or it can be done stepwise. The stepwise process follows. a. Using the equation for molality and the multiplication/division equation for random uncertainty (Appendix A ) determine the fractional uncertainty and then the absolute uncertalnty in molality. m=k77mmm= (Wint: Maltiply both sides by the molality to get the absolute uncertainty m+6m ) m=L moles/kg b. Using the equation for moles of solste and the multiplication/division equation for random uncertainty, determine the fractional uncertainty and then the absolute uncertainty in moles of solute. moles(solute)=mkg.nummolesmoles=moles(solute)= c. Repeat the process for the conversion of moles of yolute to molar mass (MM). Molar mass = 2 Maximum molar mass based on uncertainties: Minimum molar mass based on uncertainties: d. How does this uncertalnty result compare with the significant Iigure determination performed above. Explain