Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with number 4 equation 2.17 is included Part II: The Effect of Temperature on Reaction Rate Concentration of S2O32- /mol L : 0.200
please help with number 4 equation 2.17 is included 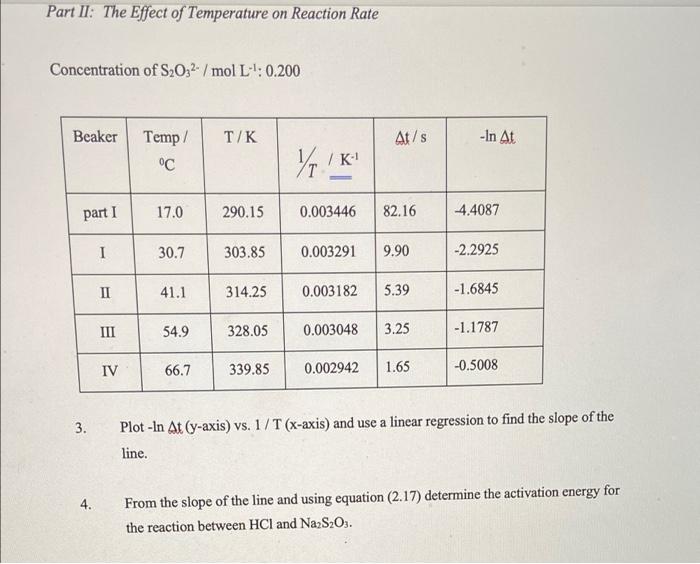
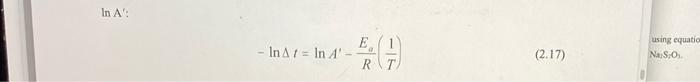
Part II: The Effect of Temperature on Reaction Rate Concentration of S2O32- /mol L : 0.200 Beaker Temp/ / At/s -In At C Yo! / K part 1 17.0 290.15 0.003446 82.16 4.4087 I 30.7 303.85 0.003291 9.90 -2.2925 II 41.1 314.25 0.003182 5.39 -1.6845 III 54.9 328.05 0.003048 3.25 -1.1787 IV 66.7 339.85 0.002942 1.65 -0.5008 3. Plot - In At (y-axis) vs. 1/T (x-axis) and use a linear regression to find the slope of the line. 4. From the slope of the line and using equation (2.17) determine the activation energy for the reaction between HCl and Na2S2O3. In A': - In A 7 = In A E 1 RT (2.17) using equatic Na S.O 
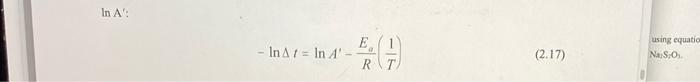
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


