Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help with prelab questions. I've attached the entire lab for context Experiment 5: The fluorometric Determination of Acetylsalicylic Acid in an Aspirin Tablet Pre-Lab
Please help with prelab questions. I've attached the entire lab for context 
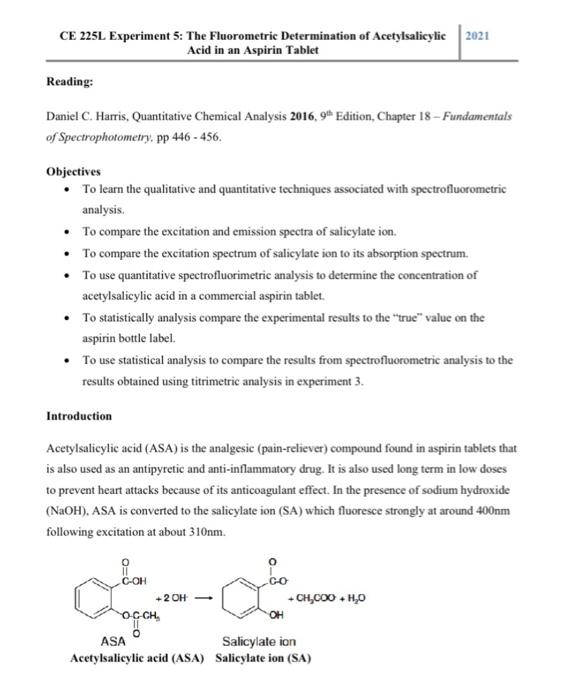





Experiment 5: The fluorometric Determination of Acetylsalicylic Acid in an Aspirin Tablet Pre-Lab Questions 1. What is the analyte in this experiment? 2. Draw the structure of salicylate ion. What part of the molecule is fluorescent? 3. Fluorescence intensity is directly proportional to concentration if certain conditions are met. What are those conditions? 4. Define fluorescence lifetime and quantum yield. 5. Describe how the excitation and emission are acquired. \begin{tabular}{l|l} CE 225L Experiment 5: The Fluorometric Determination of Acetylsalicylic & 2021 \end{tabular} Acid in an Aspirin Tablet Reading: Daniel C. Harris, Quantitative Chemical Analysis 2016, 9th Edition, Chapter 18 - Fundamentals of Spectrophotometry, pp 446 - 456. Objectives - To learn the qualitative and quantitative techniques associated with spectrofluorometric analysis. - To compare the excitation and emission spectra of salicylate ion. - To compare the excitation spectrum of salicylate ion to its absorption spectrum. - To use quantitative spectrofluorimetric analysis to determine the concentration of acetylsalicylic acid in a commercial aspirin tablet. - To statistically analysis compare the experimental results to the "true" value on the aspirin bottle label. - To use statistical analysis to compare the results from spectrofluorometric analysis to the results obtained using titrimetric analysis in experiment 3. Introduction Acetylsalicylic acid (ASA) is the analgesic (pain-reliever) compound found in aspirin tablets that is also used as an antipyretic and anti-inflammatory drug. It is also used long term in low doses to prevent heart attacks because of its anticoagulant effect. In the presence of sodium hydroxide (NaOH), ASA is converted to the salicylate ion (SA) which fluoresce strongly at around 400nm following excitation at about 310nm. \begin{tabular}{l|l} CE 225L Experiment 5: The Fluorometric Determination of Acetylsalicylic & 2021 \\ & Acid in an Aspirin Tablet \end{tabular} In addition to ASA, some aspirin tablets contain binders and buffering agents. In this experiment, portions of an aspirin tablet are dissolved in water and converted to salicylate ions by the addition of NaOH. A series of standard solutions of the SA are prepared; the fluorescence of the standards and the samples are measured; and the equation of the calibration curve is used to determine the concentration of SA in the sample solutions. The concentration is used to calculate the concentration of ASA in the solution aspirin solution and the mg ASA per tablet. The absorption, excitation and emission of SA will also be acquired and compared. Procedure: Sample Preparation Tablet samples 1. Obtain three aspirin tablets from the instructor. Record the brand name or manufacturer of the tablets. 2. Weigh three tablets separately, to the nearest 0.1mg, on an analytical balance \& label them Tab 1, Tab 2, and Tab 3. 3. Grind each tablet using a clean mortar and pestle to a fine powder. 4. From each ground tablet, weigh 3 samples - each about 0.01g (record the mass used to the nearest 0.1mg ) and label them as follows: For Tab 1: 1a, 1b, 1c For Tab 2: 2a,2b,2c For Tab 3: 3a, 3b, 3c 5. Place about 1 liter of distilled or deionized water in a 2 -liter beaker. Heat the water to just below the boiling point. 6. Fold 3 pieces of filter paper and place each in a glass funnel. Place each of the funnels on top of a 100-mL volumetric flask. Use a spray of DI water from a wash bottle to rinse the weighed tablet 1 samples from the weigh boats into the funnels. 7. Slowly pour about 70mL of hot water over each of the powder samples in the funnels. The acetylsalicylic acid slowly dissolves in the water and drains into the flask through the filter paper in the funnel. Some tablets contain binders that will not dissolve. The undissolved binder material will remain in the filter paper. Allow the solutions in the CE 225L Experiment 5: The Fluorometric Determination of Acetylsalicylic Acid in an Aspirin Tablet flasks to cool to room temperature before diluting them to the mark with roomtemperature DI water. Mix well and transfer the samples to labelled 100 -mL plastic sample bottles. 8. Repeat steps 6 and 7 twice for tablet 2 and 3 weighed powder samples. 9. Using a pipet or micropipette, transfer 1mL of each of the 100mL sample solutions in 7 and 8 to separate 10-mL volumetric flasks. Add 0.2mL of 4MNaOH using a pipet or mieropipette to each of the solutions and dilute to the mark with deionized water. Mix well and transfer to labelled 10 -mL plastic sample vials. These are your analytical samples. Standard samples 1. Weigh 0.0077g of salicylic acid to the nearest 0.1mg and quantitatively transfer weighed acid to a labeled 100-mL volumetric flask. Add about 50mL of warm DI water and swirl the flask until the solid has dissolved. Allow the solution to cool to room temperature and then dilute it to the mark with room temperature DI water. Mix well and transfer to a 100 -mL plastic sample container. This is the stock solution of salicylic acid. 2. Using a pipet or micropipette, place 0.2,0.4,0.6,0.8, and 1.0mL of the stock solution in separate and labelled 10mL volumetric flasks. Add 0.2mL of 4MNaOH to each flask and dilute to the mark using DI water. Mix well and transfer to labelled 10-mL plastic sample vials. Calculate the concentration of salicylate in each solution. These are the salicylic standard solutions. 3. Using a pipet or micropipette, place 0.2mL of 4MNaOH in a 10mL volumetric flask and dilute to the mark using DI water. Mix well. This is the blank. Analysis Excitation and Emission Spectra Fill wells A1 and A2 of a microplate with the blank and the highest concentrated standard solution, respectively. Place micro plate in the microplate holder. Well Al must be to the front left and well H1 to the right. Make sure the micro plate is well secured in place. Click on the exchange position button to move the microplate into the exchange position and the initial position button to the initial position. CE 225L Experiment 5: The Fluorometric Determination of Acetylsalicylic 2021 Acid in an Aspirin Tablet Method Creation: 1. From the method menu: Select wavelength scan as the measurement mode in the general tab. Enter the rest of the information as necessary including your name as the operator and save your method. 2. In the instrument tab: Set the scan mode (excitation or emission) and select fluorescence as the data mode. For excitation scan mode, enter the fixed wavelength on the emission side and the initial and final excitation wavelengths. For emission scan mode, enter the fixed excitation wavelength and the initial and final wavelengths. Set the rest of the parameters, which include scan speed, delay time, excitation slit, emission slit, PMT voltage, response and number of replicates. A. Excitation Spectrum. Set the Scan Start to 200nm, the Scan End to 800nm and the Emission to 400nm. Set both slits to 2.0nm. There should be a peak 310nm. B. Emission Spectrum. Set the Scan Start to 200nm, the Scan End is 800nm, the Excitation to 310nm and the Slits at 2.0nm. There should be a large peak near 400nm. Note: For the blank, there should be sharp peaks at 310nm and 400nm. 3. Monitor tab: Set the minimum and the maximum values for the ordinate and set other parameters as appropriate. 4. Processing tab: The CAT (Computing for Averaging Transient) gives an average spectrum when two or more value is entered for replicates in the instrument tab. This is good for samples with which emission intensity varies with time. Select the processing item and click the right arrow button. Select the integration method, the threshold and the sensitivity. 5. Report tab: Select options as appropriate. 6. Save your method. 7. Click the MPR icon to open the MPR setup window. Select a well to be measured and click Sample Set. To cancel a selected well, select it and click Reset. To measure a sample using an existing method, click Load MPR Method. 8. On the well information tab, enter sample name and comments as appropriate. CE 225L Experiment 5: The Fluorometric Determination of Acetylsalicylic Acid in an Aspirin Tablet 9. Select the Auto Pre-scan command from the Spectrophotometer menu. Execute pre-scan after setting the well for sample measurement. 10. Upon completion of the pre-scan task, click the Measure button. C. Absorption spectrum. Acquire the absorption spectrum of your highest SA standard on the UV-Vis spectrophotometer. Quantitative Analysis of ASA in an Aspirin Tablet Note: Carry-over contamination is a serious problem in the cuvet/well so use fresh micro plate wells. For quantitative analysis, it is best to start with the blank solution and acquire the standards in the order of increasing concentration. Each well must be filled with 400L of sample. 1. Click the Method icon/button 2. General tab: Select photometry as the measurement method, enter your name as the operator and add comments as appropriate. 3. Quantitation tab: Select Wavelength as the Quantitation type, 1R order as the Calibration type, 1 as Number of wavelengths and enter the rest of the information as appropriate. 4. Instrument tab: Select Fluorescence as the Data mode, Both WL Fixed as the Wavelength mode, 5.0nm slit with for both EX and EM, PMT voltage 700V, and 0.1s integration time. Enter the rest of the information as appropriate. 5. Monitor tab: Enter values as appropriate. Check the Open data processing window after data acquisition. 6. Report tab: Select options as appropriate. 7. Save your method. 8. Click the MPR icon to open the MPR setup window. Select a well to be measured and click Standard Set. Wells should be standard-specified sequentially starting from well A1. Select a well to be measured and click Sample Set. To cancel a selected well, select it and click Reset. To measure a sample using an existing method, click Load MPR Method. 9. On the well information tab, enter (i) standards and their concentrations and (ii) sample labels. Acid in an Aspirin Tablet 10. Fill wells A1 to A6 with your standard solutions (400L) in ascending order (Al blank). Place your samples (400L) in the subsequent cells. Place micro plate on the microplate holder. Well Al must be to the front left and well H1 to the right. Make sure the micro plate is well secured in place. Load the micro plate, bring the monitor window to the foremost, and click the Measure button. Measure each sample three times. Data Analysis 1. Generate an overlay graph containing the excitation and emission spectra of the SA standard and the blank. Annotate the peak excitation and peak emission wavelengths. Identify and label the Rayleigh and Raman (if visible) scattering peaks. Compare the excitation spectrum to the emission spectrum and the absorption spectrum. 2. From the quantitative data, calculate the percentage of ASA in the tablet. Report your answer as avg. std. dev. 3. Calculate the mg of ASA per tablet. Report your answer as avg. std. dev. 4. Use the student-t test to compare your results (mg ASA per tablet) to the mg of ASA on the vitamin bottle label. 5. Use the student-t test to compare your results to the results you obtained in experiment 3 (titrimetric analysis of ASA ). 6. Use your quantitative data to calculate S/N ratio 






Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


