Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help with the last two tables and the questions ... For table 4, the data in the table has to be derived from those
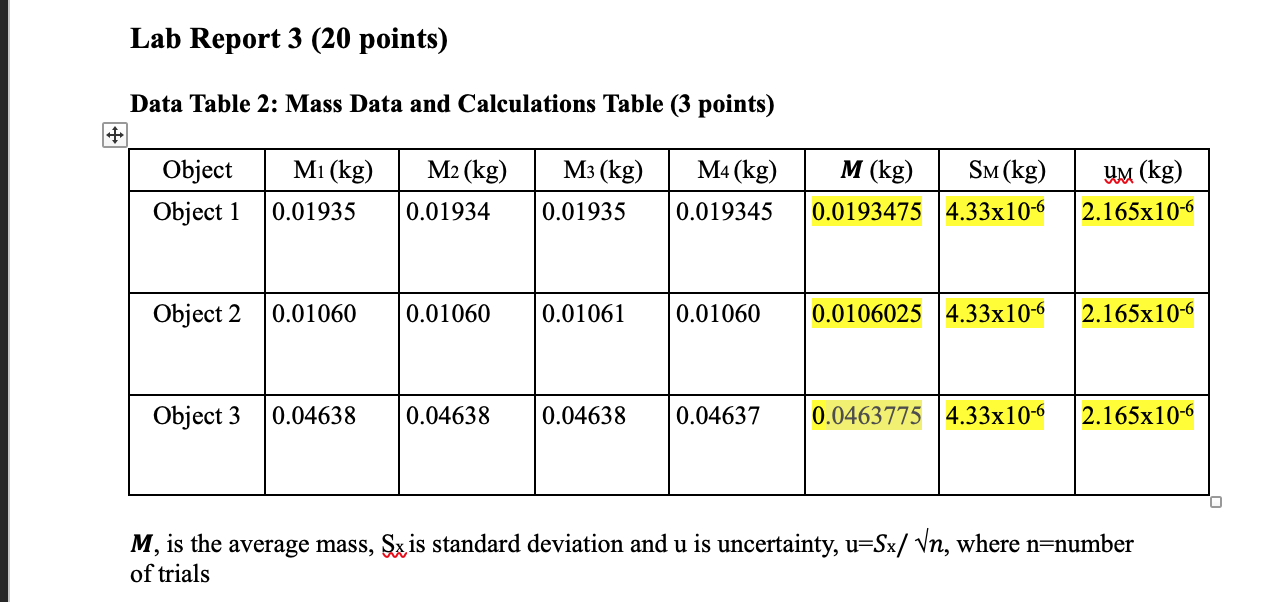
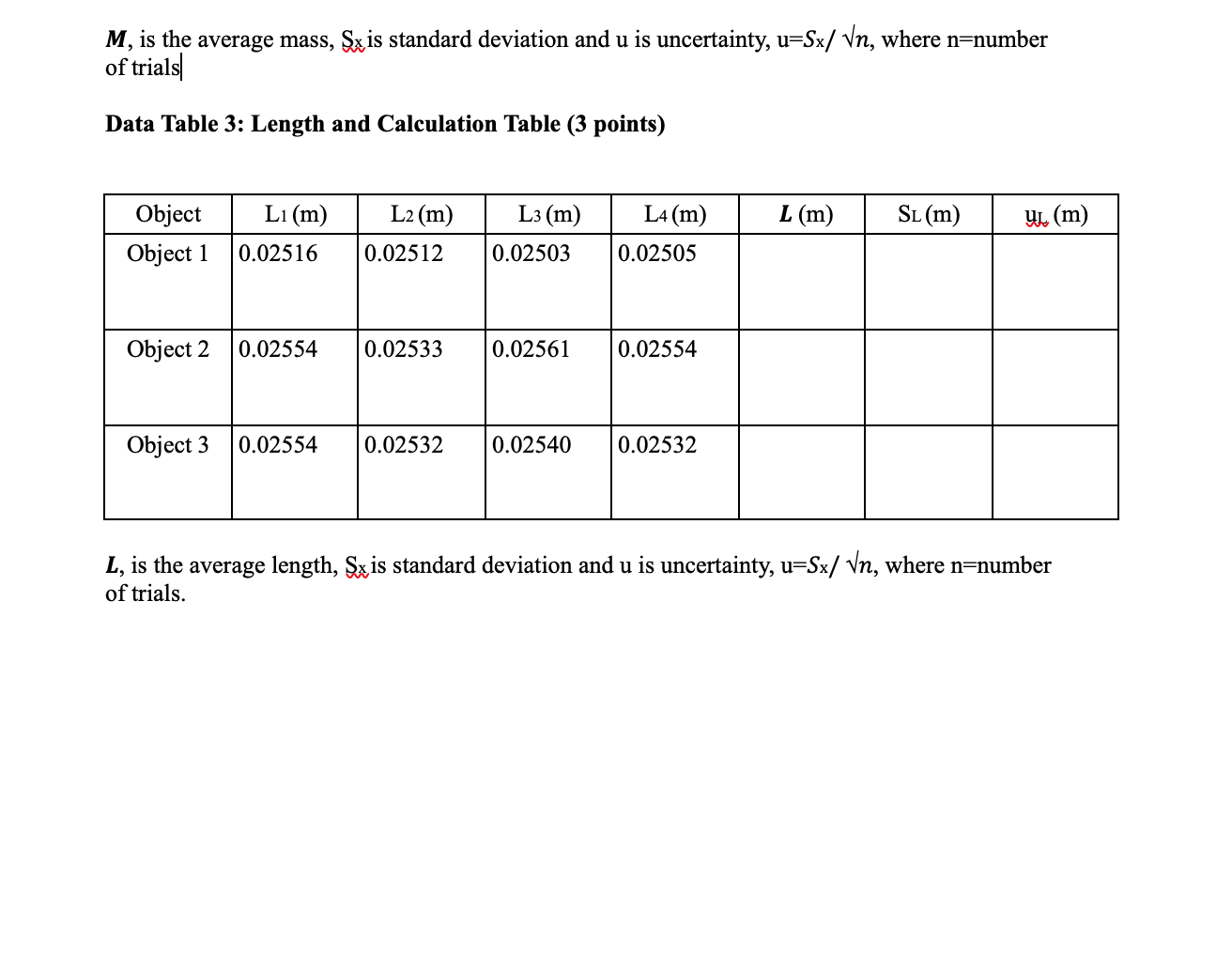


Please help with the last two tables and the questions ... For table 4, the data in the table has to be derived from those tables in 2 and 3.
Lab Report 3 (20 points) Data Table 2: Mass Data and Calculations Table (3 points) Mi(kg) M2(kg) M3 (kg) M4(kg) Object Object 1 0.01935 M (kg) SM (kg) 0.0193475 4.33x10-6 UM (kg) 2.165x10-6 0.01934 0.01935 0.019345 Object 2 0.01060 0.01060 0.01061 0.01060 0.0106025 4.33x10-6 2.165x10-6 Object 3 0.04638 0.04638 0.04638 0.04637 0.0463775 4.33x10-6 2.165x10-6 M, is the average mass, Sx is standard deviation and u is uncertainty, u=Sx/ Vn, where n=number of trials M, is the average mass, Sx is standard deviation and u is uncertainty, u=Sx/ Vn, where n=number of trials Data Table 3: Length and Calculation Table (3 points) L1 (m) L2(m) L3 (m) L (m) Sl(m) Object Object 1 L4 (m) 0.02505 9 (m) 0.02516 0.02512 0.02503 Object 2 0.02554 0.02533 0.02561 0.02554 Object 3 0.02554 0.02532 0.02540 0.02532 L, is the average length, Sx is standard deviation and u is uncertainty, u=Sx/ Vn, where n=number of trials. Header Data Table 4: Volume and Density Calculations (5 points) Material % error Object Volume = Ux=3LuL Density ( ) = up L3 (m2) (units 2 M/V (kg/m) (units= Object Standard Density (kg/m3) Object 2. Object 3 Questions: 1. Which object has the most accurate determination of the density of the material? Show clearly how you determine the accuracy of your evaluations of the density. (1 point) 2. Which object has the most precise determination of the density of the material? Show clearly how you determine the precision of your evaluations of the density. (1 point) 3. Which object has the most accurate measurement of M? Explain. (1 point) 4. Which object has the most precise measurement of M? Explain. (1 point) Header 5. Which object has the most accurate measurement of L? Explain. (1 point) 6. Which object has the most precise measurement of L? Explain. (1 point) 7. Based on the values of the uncertainties for M and L (and the formulas for ux and up), which one do you think was contributing more to the uncertainty in the density? Explain. (1 point) 8. Based on your experimental data, which one between M and L most affected the accuracy of the density? Clearly state your reasoning (hint: what assumption have we made?). (1 point) 9. Can you suggest an alternative method to evaluate the density and what would its advantage be? (1 point) Lab Report 3 (20 points) Data Table 2: Mass Data and Calculations Table (3 points) Mi(kg) M2(kg) M3 (kg) M4(kg) Object Object 1 0.01935 M (kg) SM (kg) 0.0193475 4.33x10-6 UM (kg) 2.165x10-6 0.01934 0.01935 0.019345 Object 2 0.01060 0.01060 0.01061 0.01060 0.0106025 4.33x10-6 2.165x10-6 Object 3 0.04638 0.04638 0.04638 0.04637 0.0463775 4.33x10-6 2.165x10-6 M, is the average mass, Sx is standard deviation and u is uncertainty, u=Sx/ Vn, where n=number of trials M, is the average mass, Sx is standard deviation and u is uncertainty, u=Sx/ Vn, where n=number of trials Data Table 3: Length and Calculation Table (3 points) L1 (m) L2(m) L3 (m) L (m) Sl(m) Object Object 1 L4 (m) 0.02505 9 (m) 0.02516 0.02512 0.02503 Object 2 0.02554 0.02533 0.02561 0.02554 Object 3 0.02554 0.02532 0.02540 0.02532 L, is the average length, Sx is standard deviation and u is uncertainty, u=Sx/ Vn, where n=number of trials. Header Data Table 4: Volume and Density Calculations (5 points) Material % error Object Volume = Ux=3LuL Density ( ) = up L3 (m2) (units 2 M/V (kg/m) (units= Object Standard Density (kg/m3) Object 2. Object 3 Questions: 1. Which object has the most accurate determination of the density of the material? Show clearly how you determine the accuracy of your evaluations of the density. (1 point) 2. Which object has the most precise determination of the density of the material? Show clearly how you determine the precision of your evaluations of the density. (1 point) 3. Which object has the most accurate measurement of M? Explain. (1 point) 4. Which object has the most precise measurement of M? Explain. (1 point) Header 5. Which object has the most accurate measurement of L? Explain. (1 point) 6. Which object has the most precise measurement of L? Explain. (1 point) 7. Based on the values of the uncertainties for M and L (and the formulas for ux and up), which one do you think was contributing more to the uncertainty in the density? Explain. (1 point) 8. Based on your experimental data, which one between M and L most affected the accuracy of the density? Clearly state your reasoning (hint: what assumption have we made?). (1 point) 9. Can you suggest an alternative method to evaluate the density and what would its advantage be? (1 point)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


