Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help with this three questions 5g of calcium carbonate takes 250s to react with hydrochloric acid completely. What is the average rate of the
Please help with this three questions 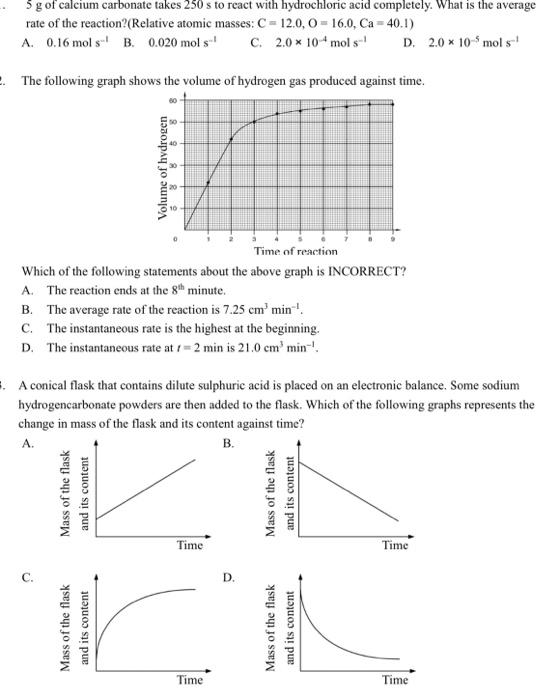
5g of calcium carbonate takes 250s to react with hydrochloric acid completely. What is the average rate of the reaction?(Relative atomic masses: C=12.0,O=16.0,Ca=40.1 ) A. 0.16mols1 B. 0.020mols1 C. 2.0104mols1 D. 2.0105mols1 The following graph shows the volume of hydrogen gas produced against time. Which of the following statements about the above graph is INCORRECT? A. The reaction ends at the 8th minute. B. The average rate of the reaction is 7.25cm3min1. C. The instantaneous rate is the highest at the beginning. D. The instantaneous rate at t=2min is 21.0cm3min1. A conical flask that contains dilute sulphuric acid is placed on an electronic balance. Some sodium hydrogencarbonate powders are then added to the flask. Which of the following graphs represents the change in mass of the flask and its content against time? A. B. C. D 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


