Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please i only need calculations for trial 1 only for 1. Heat realeased by liquid 2. Heat absorbed by solid 3. Specific enthalpy of fusion
please i only need calculations for trial 1 only for
18,2969 0.83 Your report should include these report sheets, responses to questions, and copies of your three calorimetry runs. Be sure to include units where appropriate and report values to the correct number of significant figures. Trial 1 Trial 2 Trial 3 Mass of calorimeter (cups + stir bar) 10.5249 10.5249 (0.5249 Mass of calorimeter + liquid 88.826989.6969 96.2479 Mass of calorimeter + liquid + melted solid 108.984 9107.3219 108.7999 Mass of liquid (m) 79.1729 79.7239 Mass of solid (ms) 19.9589 17. 625g 18.5529 Melting temperature (Tm) 10.83C 10,83 Initial temperature (T) 24.26 & 25.000 25.380 C Time ice added to water (1) 2.35 min 2.255 min 2.817 min Slope of linear fit (m) 10.2039, 40.1759 dmin (0.1816 cl mis Intercept of linear fit (b) 5.612C 14.905C Final temperature (T) 3.47c 6.01 5.51C Heat released by liquid (9) ? Heat absorbed by solid (9) ? Specific enthalpy of fusion P (.) Molar enthalpy of fusion ? (AHtus) Average molar enthalpy of fusion (AHM) 2.978 18,2969 0.83 Your report should include these report sheets, responses to questions, and copies of your three calorimetry runs. Be sure to include units where appropriate and report values to the correct number of significant figures. Trial 1 Trial 2 Trial 3 Mass of calorimeter (cups + stir bar) 10.5249 10.5249 (0.5249 Mass of calorimeter + liquid 88.826989.6969 96.2479 Mass of calorimeter + liquid + melted solid 108.984 9107.3219 108.7999 Mass of liquid (m) 79.1729 79.7239 Mass of solid (ms) 19.9589 17. 625g 18.5529 Melting temperature (Tm) 10.83C 10,83 Initial temperature (T) 24.26 & 25.000 25.380 C Time ice added to water (1) 2.35 min 2.255 min 2.817 min Slope of linear fit (m) 10.2039, 40.1759 dmin (0.1816 cl mis Intercept of linear fit (b) 5.612C 14.905C Final temperature (T) 3.47c 6.01 5.51C Heat released by liquid (9) ? Heat absorbed by solid (9) ? Specific enthalpy of fusion P (.) Molar enthalpy of fusion ? (AHtus) Average molar enthalpy of fusion (AHM) 2.978 1. Heat realeased by liquid
2. Heat absorbed by solid
3. Specific enthalpy of fusion
4. Molar enthalpy of fusion.
Thank you so much!! 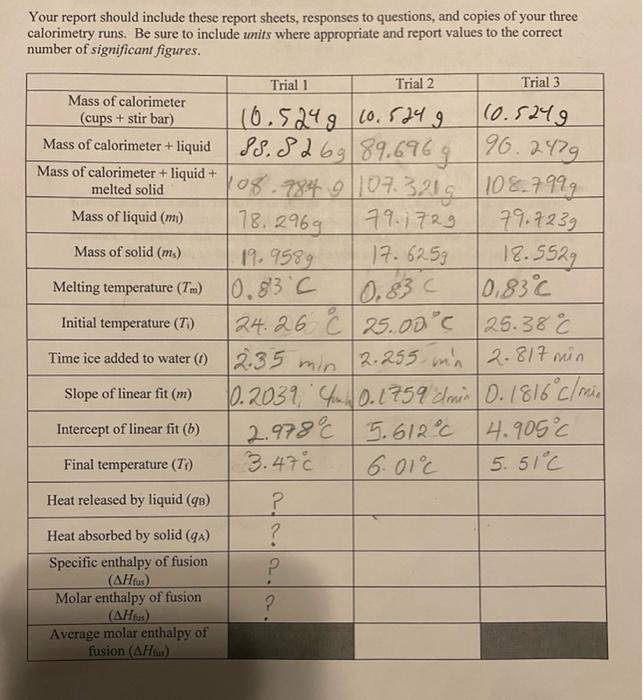

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


