Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please make sure to answer bottom problem as well! thank you At 2.12 atm and 504 K, what volume of NO, gas forms from 43,40
please make sure to answer bottom problem as well! thank you 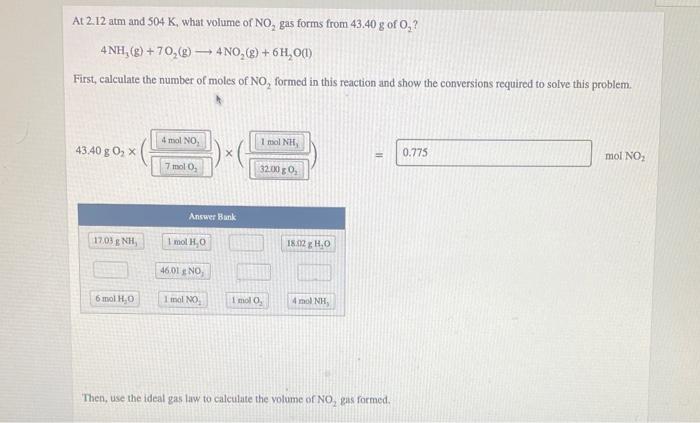
At 2.12 atm and 504 K, what volume of NO, gas forms from 43,40 g of O,? 4NH,(8) + 70,(g) 4NO,(g) + 6H,00) First, calculate the number of moles of NO, formed in this reaction and show the conversions required to solve this problem. 4 mol NO 1 mol NH 43.40 g 0 x 3) 0.775 Il mol NO: 7 molo 320039 Answer Bank 1703 ENH, 1 mol Ho 18.12 H.O 46 01 NO 6 mol H2O 1 mol NO i molo 4 mol NH Then, use the ideal gas law to calculate the volume of NO, gas formed 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


