Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please may you kindly assist with 1 (c,d,e) and 2 (a-c) I am struggling to get the correct answers . Note: The Antoine constants given
please may you kindly assist with 1 (c,d,e) and 2 (a-c) I am struggling to get the correct answers .
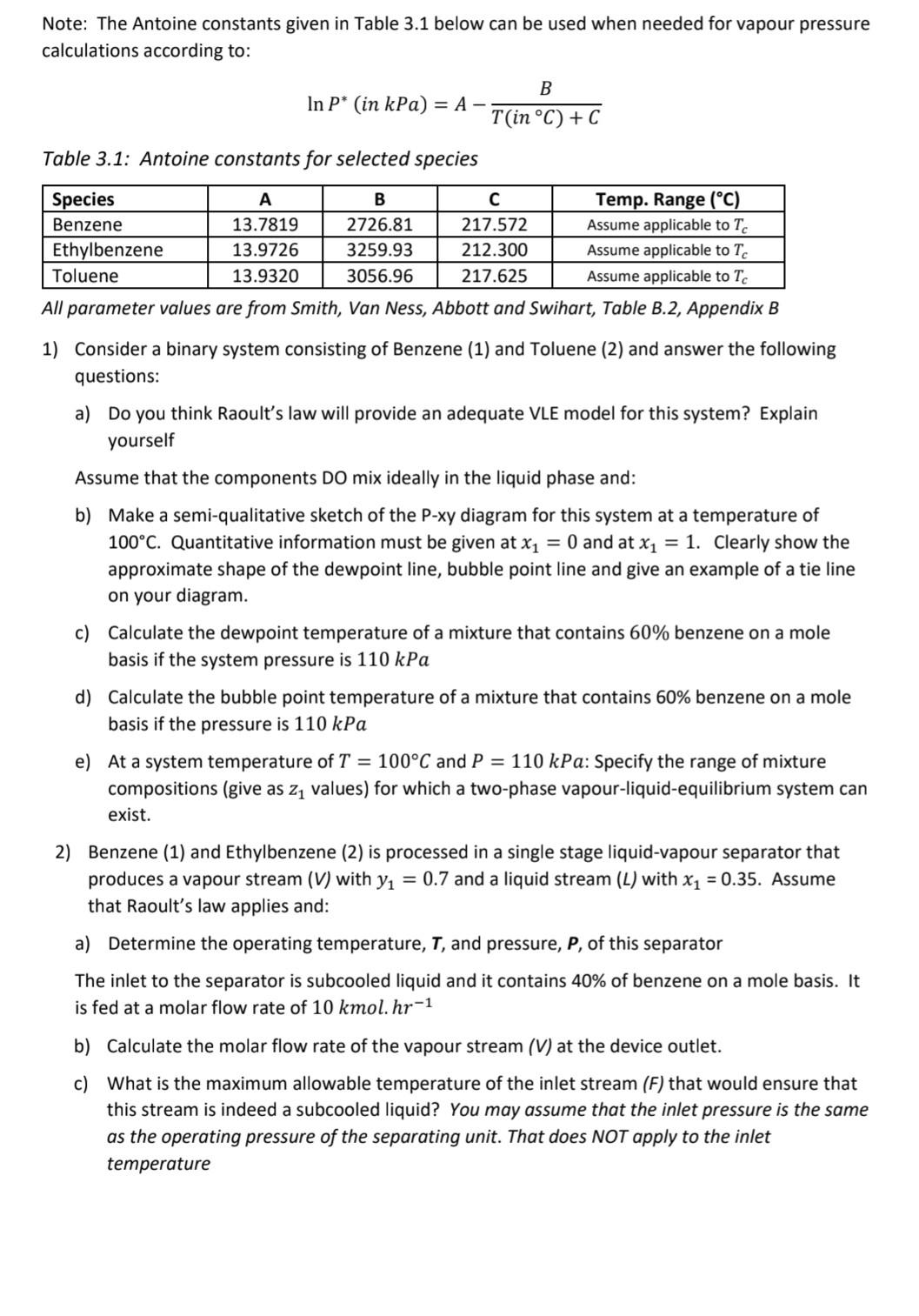
Note: The Antoine constants given in Table 3.1 below can be used when needed for vapour pressure calculations according to: lnP(inkPa)=AT(inC)+CB Table 3.1: Antoine constants for selected species All parameter values are from Smith, Van Ness, Abbott and Swihart, Table B.2, Appendix B 1) Consider a binary system consisting of Benzene (1) and Toluene (2) and answer the following questions: a) Do you think Raoult's law will provide an adequate VLE model for this system? Explain yourself Assume that the components DO mix ideally in the liquid phase and: b) Make a semi-qualitative sketch of the P-xy diagram for this system at a temperature of 100C. Quantitative information must be given at x1=0 and at x1=1. Clearly show the approximate shape of the dewpoint line, bubble point line and give an example of a tie line on your diagram. c) Calculate the dewpoint temperature of a mixture that contains 60% benzene on a mole basis if the system pressure is 110kPa d) Calculate the bubble point temperature of a mixture that contains 60% benzene on a mole basis if the pressure is 110kPa e) At a system temperature of T=100C and P=110kPa : Specify the range of mixture compositions (give as z1 values) for which a two-phase vapour-liquid-equilibrium system can exist. 2) Benzene (1) and Ethylbenzene (2) is processed in a single stage liquid-vapour separator that produces a vapour stream (V) with y1=0.7 and a liquid stream (L) with x1=0.35. Assume that Raoult's law applies and: a) Determine the operating temperature, T, and pressure, P, of this separator The inlet to the separator is subcooled liquid and it contains 40% of benzene on a mole basis. It b) Calculate the molar flow rate of the vapour stream (V) at the device outlet. c) What is the maximum allowable temperature of the inlet stream (F) that would ensure that this stream is indeed a subcooled liquid? You may assume that the inlet pressure is the same as the operating pressure of the separating unit. That does NOT apply to the inlet temperature Note: The Antoine constants given in Table 3.1 below can be used when needed for vapour pressure calculations according to: lnP(inkPa)=AT(inC)+CB Table 3.1: Antoine constants for selected species All parameter values are from Smith, Van Ness, Abbott and Swihart, Table B.2, Appendix B 1) Consider a binary system consisting of Benzene (1) and Toluene (2) and answer the following questions: a) Do you think Raoult's law will provide an adequate VLE model for this system? Explain yourself Assume that the components DO mix ideally in the liquid phase and: b) Make a semi-qualitative sketch of the P-xy diagram for this system at a temperature of 100C. Quantitative information must be given at x1=0 and at x1=1. Clearly show the approximate shape of the dewpoint line, bubble point line and give an example of a tie line on your diagram. c) Calculate the dewpoint temperature of a mixture that contains 60% benzene on a mole basis if the system pressure is 110kPa d) Calculate the bubble point temperature of a mixture that contains 60% benzene on a mole basis if the pressure is 110kPa e) At a system temperature of T=100C and P=110kPa : Specify the range of mixture compositions (give as z1 values) for which a two-phase vapour-liquid-equilibrium system can exist. 2) Benzene (1) and Ethylbenzene (2) is processed in a single stage liquid-vapour separator that produces a vapour stream (V) with y1=0.7 and a liquid stream (L) with x1=0.35. Assume that Raoult's law applies and: a) Determine the operating temperature, T, and pressure, P, of this separator The inlet to the separator is subcooled liquid and it contains 40% of benzene on a mole basis. It b) Calculate the molar flow rate of the vapour stream (V) at the device outlet. c) What is the maximum allowable temperature of the inlet stream (F) that would ensure that this stream is indeed a subcooled liquid? You may assume that the inlet pressure is the same as the operating pressure of the separating unit. That does NOT apply to the inlet temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


