Question
Determine which base will work to deprotonate each compound in an acid/base extraction. OH Choose... H3C- HC CH3 H2 H2 Choose... OH H2 OH
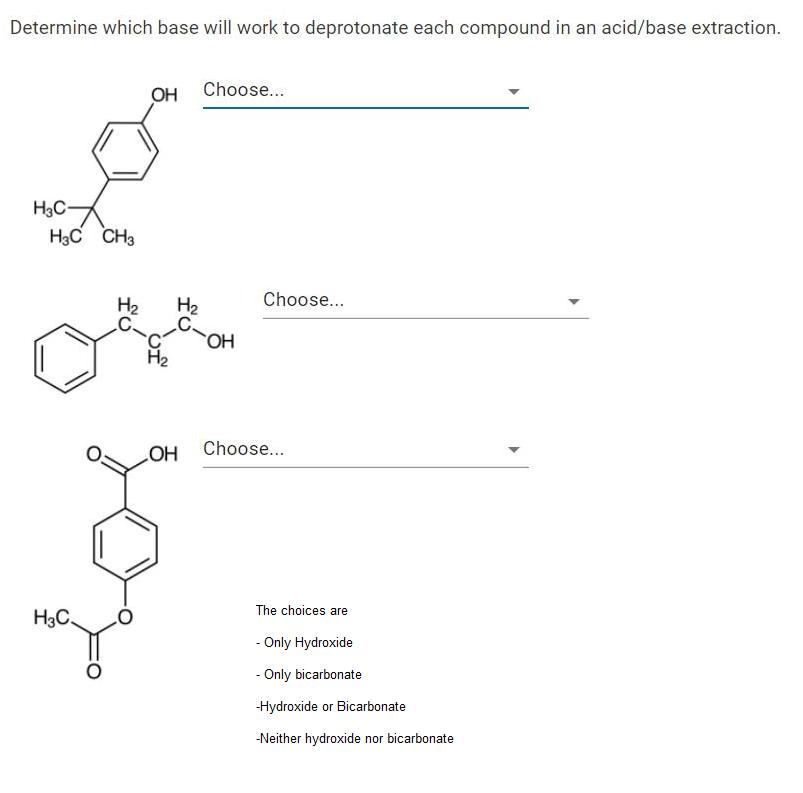
Determine which base will work to deprotonate each compound in an acid/base extraction. OH Choose... H3C- HC CH3 H2 H2 Choose... OH H2 OH Choose.. The choices are H3C. - Only Hydroxide - Only bicarbonate Hydroxide or Bicarbonate -Neither hydroxide nor bicarbonate
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Solu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus Early Transcendentals
Authors: James Stewart
7th edition
538497904, 978-0538497909
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



