Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE PLEASE PLEASE DO NOT COPY AND PASTE SOLUTION FROM A DIFFERENT QUESTION, THIS IS NOT ASKING FOR THE SAME THING. please use Matlab to
 PLEASE
PLEASE
PLEASE
PLEASE DO NOT COPY AND PASTE SOLUTION FROM A DIFFERENT QUESTION, THIS IS NOT ASKING FOR THE SAME THING.
please use Matlab to solve the nonlinear equation
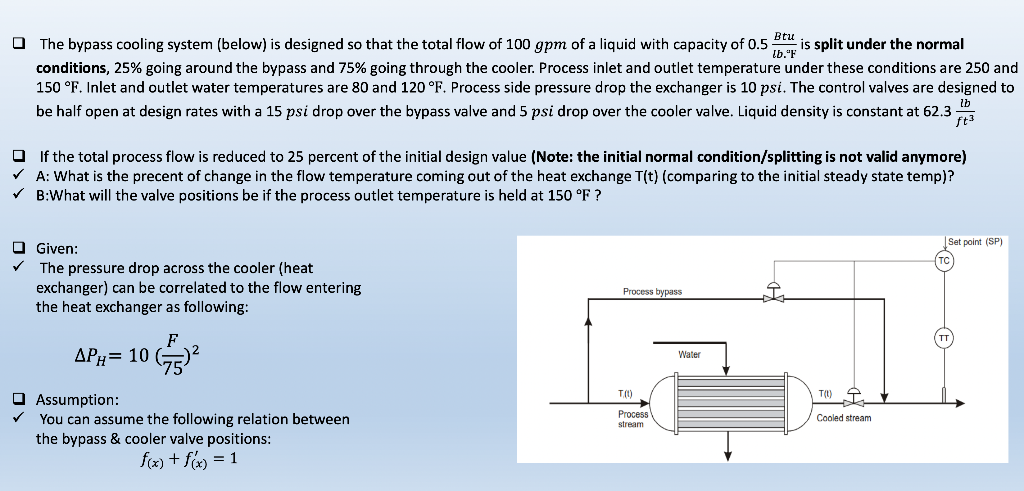
The bypass cooling system (below) is designed so that the total flow of 100 gpm of a liquid with capacity of 0.5 Btu is split under the normal conditions, 25% going around the bypass and 75% going through the cooler. Process inlet and outlet temperature under these conditions are 250 and lb." 150 F. Inlet and outlet water temperatures are 80 and 120 F. Process side pressure drop the exchanger is 10 psi. The control valves are designed to be half open at design rates with a 15 psi drop over the bypass valve and 5 psi drop over the cooler valve. Liquid density is constant at 62.3 lb ft If the total process flow is reduced to 25 percent of the initial design value (Note: the initial normal condition/splitting is not valid anymore) A: What is the precent of change in the flow temperature coming out of the heat exchange T(t) (comparing to the initial steady state temp)? B:What will the valve positions be if the process outlet temperature is held at 150 F ? Set point (SP) Given: The pressure drop across the cooler (heat exchanger) can be correlated to the flow entering the heat exchanger as following: Process bypass F AP = 10652 Water T(U) TOI Cooled stream Process stream Assumption: You can assume the following relation between the bypass & cooler valve positions: f(x) + f(x) = 1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


