Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please provide me with the MATLAB code. It's Che 321 class. Otherwise, you are not helping me. Thank you very much. (40 points) MATLAB practice!

Please provide me with the MATLAB code. It's Che 321 class. Otherwise, you are not helping me. Thank you very much.
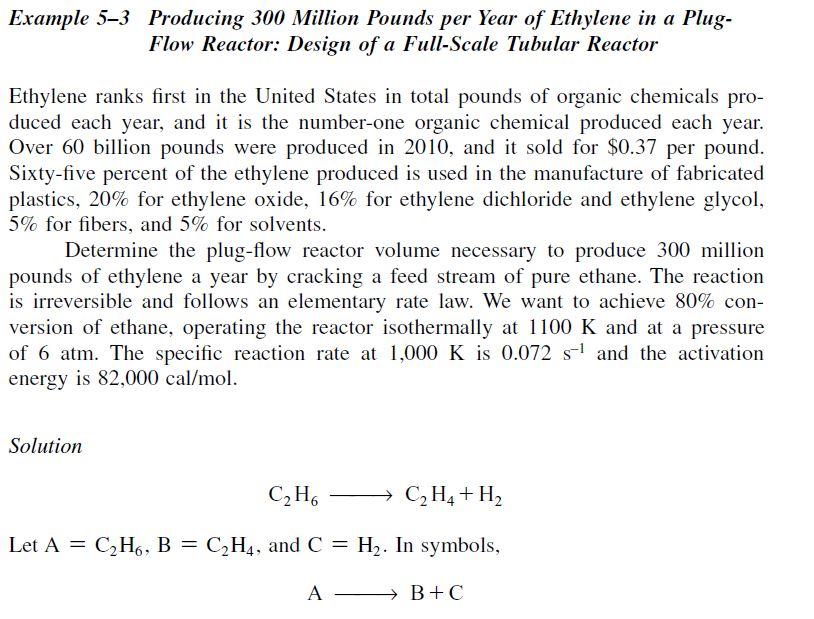
(40 points) MATLAB practice! Example 5-3 in the 5th edition of the fogler text has a great example of calculating a production scale tubular reactor. I would like you all to solve the example in matlab and do your best to reproduce the concentration/conversion profiles in figure E5-3.1. All of the derivations have been completed, it is your task to transfer it into code (excel is fine here but it will not be your friend once coupled ODE's enter the picture, this is a simple separable solution). Please do not do this by hand (it also won't be accepted) as the simple coupled equations will give you a worse headache than a teething toddler at 3am. Please provide your code (workbook, marcros, etc.), outputs, and chart. Example 5-3 Producing 300 Million Pounds per Year of Ethylene in a PlugFlow Reactor: Design of a Full-Scale Tubular Reactor Ethylene ranks first in the United States in total pounds of organic chemicals produced each year, and it is the number-one organic chemical produced each year. Over 60 billion pounds were produced in 2010, and it sold for $0.37 per pound. Sixty-five percent of the ethylene produced is used in the manufacture of fabricated plastics, 20% for ethylene oxide, 16% for ethylene dichloride and ethylene glycol, 5% for fibers, and 5% for solvents. Determine the plug-flow reactor volume necessary to produce 300 million pounds of ethylene a year by cracking a feed stream of pure ethane. The reaction is irreversible and follows an elementary rate law. We want to achieve 80% conversion of ethane, operating the reactor isothermally at 1100K and at a pressure of 6atm. The specific reaction rate at 1,000K is 0.072s1 and the activation energy is 82,000cal/mol. Solution C2H6C2H4+H2 Let A=C2H6,B=C2H4, and C=H2. In symbols, AB+CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


