Answered step by step
Verified Expert Solution
Question
1 Approved Answer
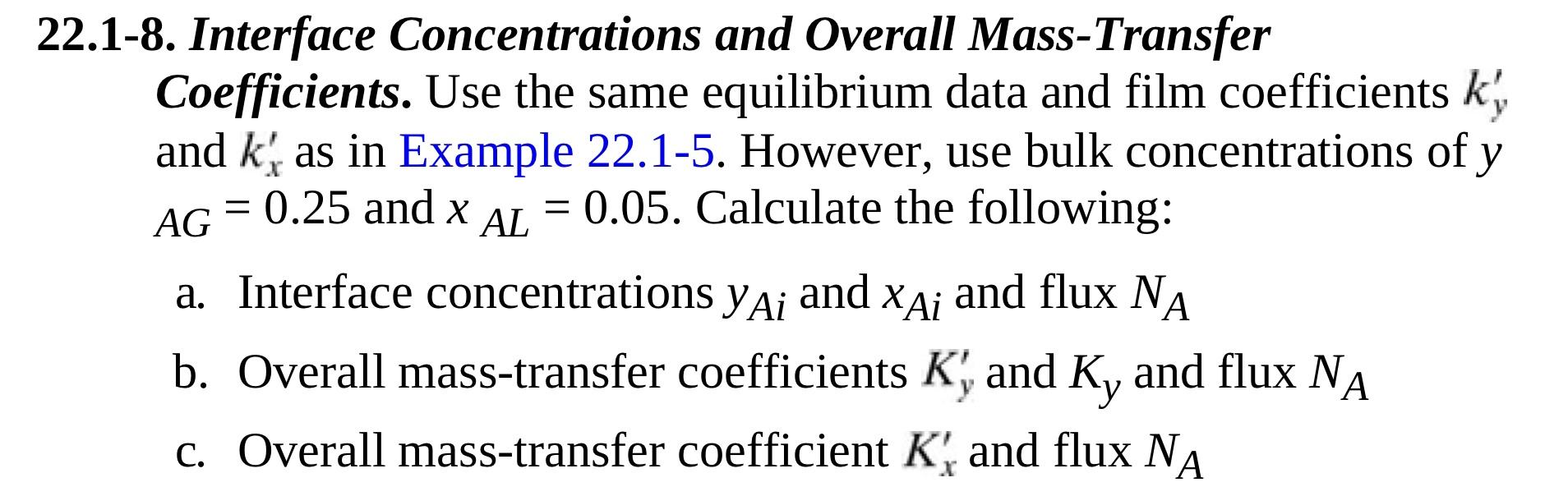
Please see below for example 22.1-5, but only answer 22.1-8 thanks! 2.1-8. Interface Concentrations and Overall Mass-Transfer Coefficients. Use the same equilibrium data and film
Please see below for example 22.1-5, but only answer 22.1-8 thanks!
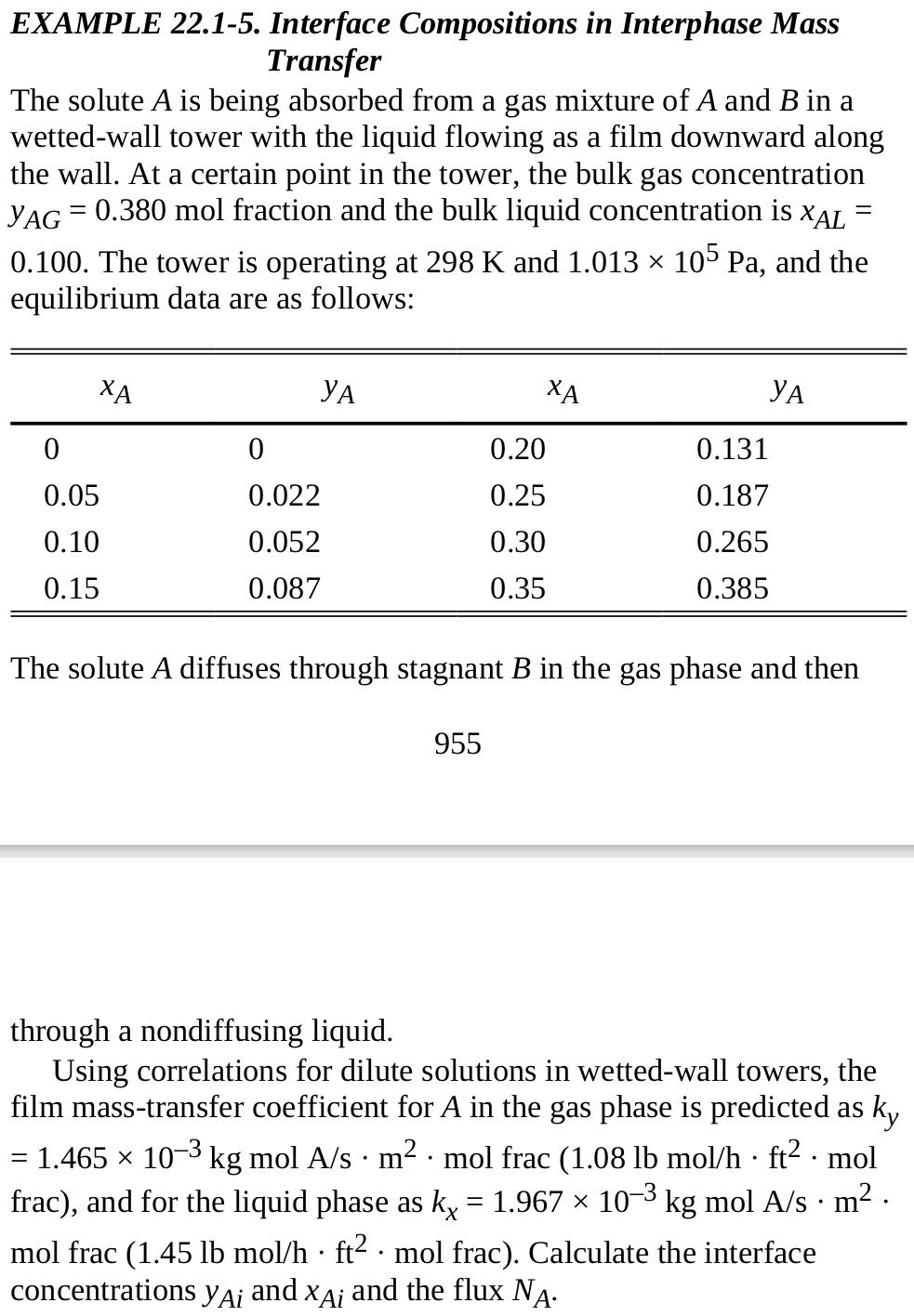
2.1-8. Interface Concentrations and Overall Mass-Transfer Coefficients. Use the same equilibrium data and film coefficients ky and kx as in Example 22.1-5. However, use bulk concentrations of y AG=0.25 and xAL=0.05. Calculate the following: a. Interface concentrations yAi and xAi and flux NA b. Overall mass-transfer coefficients Ky and Ky and flux NA c. Overall mass-transfer coefficient Kx and flux NA EXAMPLE 22.1-5. Interface Compositions in Interphase Mass Transfer The solute A is being absorbed from a gas mixture of A and B in a wetted-wall tower with the liquid flowing as a film downward along the wall. At a certain point in the tower, the bulk gas concentration yAG=0.380mol fraction and the bulk liquid concentration is xAL= 0.100. The tower is operating at 298K and 1.013105Pa, and the equilibrium data are as follows: The solute A diffuses through stagnant B in the gas phase and then 955 through a nondiffusing liquid. Using correlations for dilute solutions in wetted-wall towers, the film mass-transfer coefficient for A in the gas phase is predicted as ky =1.465103kgmolA/sm2molfrac(1.08lbmol/hft2mol frac), and for the liquid phase as kx=1.967103kgmolA/sm2. mol frac (1.45lbmol/hft2mol frac). Calculate the interface concentrations yAi and xAi and the flux NA
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


