Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please share each calculation and share the graphs so I can learn how to prepare this type of answer. A process fills cans with cola
Please share each calculation and share the graphs so I can learn how to prepare this type of answer.
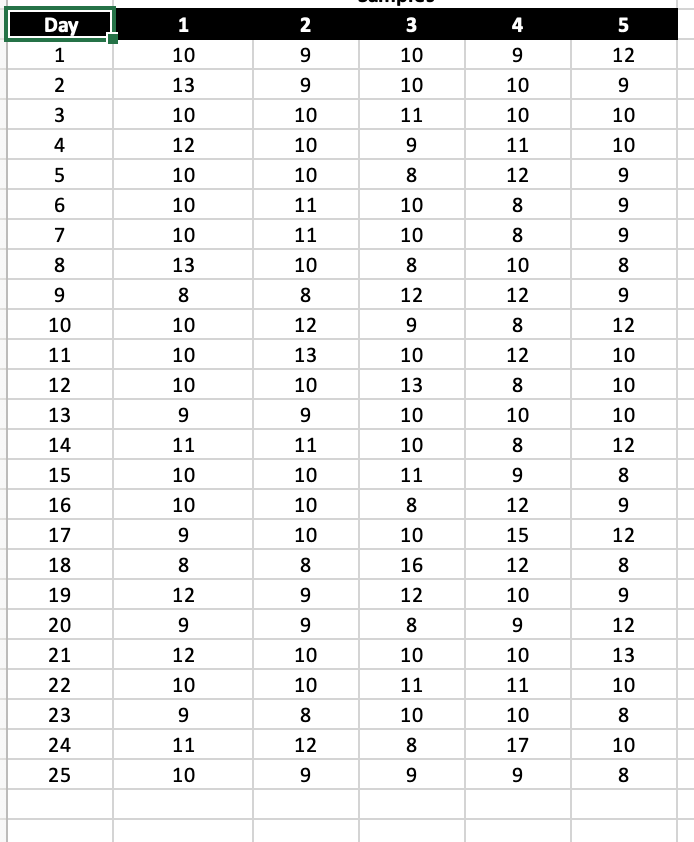
A process fills cans with cola and the cans must have the right amount of product before shipping to the wholesaler. Samples are taken over 25 days, and the amount of cola in ounces is measured. The aim is to achieve statistical control of the filling process using X and R-charts.
1
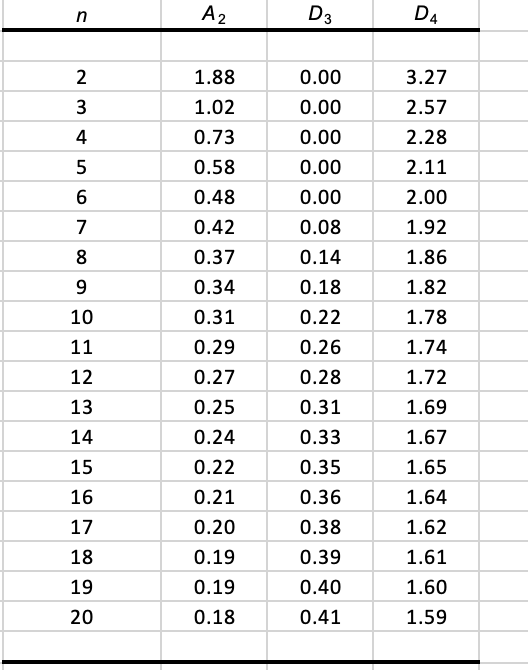
Prepare X and R-charts using these data with the standard 3-sigma control limit constants A2, D3, and D4 (see the Factors worksheet).
\begin{tabular}{|c|c|c|c|} \hlinen & A2 & D3 & D4 \\ \hline & & & \\ \hline 2 & 1.88 & 0.00 & 3.27 \\ \hline 3 & 1.02 & 0.00 & 2.57 \\ \hline 4 & 0.73 & 0.00 & 2.28 \\ \hline 5 & 0.58 & 0.00 & 2.11 \\ \hline 6 & 0.48 & 0.00 & 2.00 \\ \hline 7 & 0.42 & 0.08 & 1.92 \\ \hline 8 & 0.37 & 0.14 & 1.86 \\ \hline 9 & 0.34 & 0.18 & 1.82 \\ \hline 10 & 0.31 & 0.22 & 1.78 \\ \hline 11 & 0.29 & 0.26 & 1.74 \\ \hline 12 & 0.27 & 0.28 & 1.72 \\ \hline 13 & 0.25 & 0.31 & 1.69 \\ \hline 14 & 0.24 & 0.33 & 1.67 \\ \hline 15 & 0.22 & 0.35 & 1.65 \\ \hline 16 & 0.21 & 0.36 & 1.64 \\ \hline 17 & 0.20 & 0.38 & 1.62 \\ \hline 18 & 0.19 & 0.39 & 1.61 \\ \hline 19 & 0.19 & 0.40 & 1.60 \\ \hline 20 & 0.18 & 0.41 & 1.59 \\ \hline & & & \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


