Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please Show All Steps I think I need to use Free energy = (3/2)kBT((R^2)/(Nb^2) but am not sure Consider a randomly branched polymer in a
Please Show All Steps
I think I need to use Free energy = (3/2)kBT((R^2)/(Nb^2) but am not sure
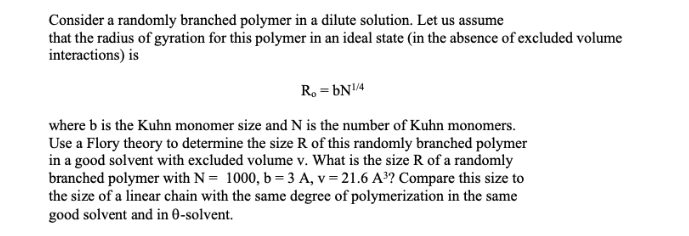
Consider a randomly branched polymer in a dilute solution. Let us assume that the radius of gyration for this polymer in an ideal state (in the absence of excluded volume interactions) is R0=bN1/4 where b is the Kuhn monomer size and N is the number of Kuhn monomers. Use a Flory theory to determine the size R of this randomly branched polymer in a good solvent with excluded volume v. What is the size R of a randomly branched polymer with N=1000,b=3A,v=21.6A3 ? Compare this size to the size of a linear chain with the same degree of polymerization in the same good solvent and in -solventStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


