Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show all work if possible 3. A stream containing H2S and inert gases and a second stream of pure SO2 are fed to a
please show all work if possible 
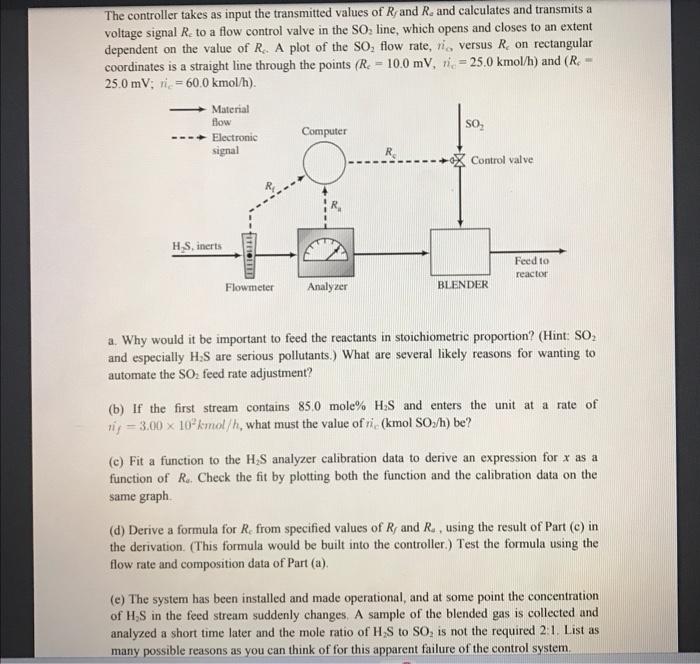
3. A stream containing H2S and inert gases and a second stream of pure SO2 are fed to a sulfur recovery reactor, where the reaction 2H2S+SO2+3S+2H2O takes place. The feed rates are adjusted so that the ratio of H2S to SO2 in the combined feed is always stoichiometric In the normal operation of the reactor the flow rate and composition of the H2S feed stream both fluctuate. In the past, each time either variable changed the required SO2 feed rate had to be reset by adjusting a valve in the feed line. A control system has been installed to automate this process. The H2S feed stream passes through an electronic flowmeter that transmits a signal Rf directly proportional to the molar flow rate of the stream, nj. When nf=100 kmol/h, the transmitted signal Rf=15mV. The mole fraction of H2S in this stream is measured with a thermal conductivity detector, which transmits a signal Ra. Analyzer calibration data are as follows: The controller takes as input the transmitted values of R and Ra and calculates and transmits a voltage signal Rc to a flow control valve in the SO2 line, which opens and closes to an extent dependent on the value of Rc. A plot of the SO2 flow rate, nc, versus Rc on rectangular coordinates is a straight line through the points (Rc=10.0mV,nc=25.0kmol/h) and (Rc= 25.0mV;nc=60.0kmol/h). a. Why would it be important to feed the reactants in stoichiometric proportion? (Hint: SO2 and especially H2S are serious pollutants.) What are several likely reasons for wanting to automate the SO2 feed rate adjustment? (b) If the first stream contains 85.0 mole %H2S and enters the unit at a rate of (c) Fit a function to the H2S analyzer calibration data to derive an expression for x as a function of Ra. Check the fit by plotting both the function and the calibration data on the same graph. (d) Derive a formula for Rc from specified values of Rf and Ra, using the result of Part (c) in the derivation. (This formula would be built into the controller.) Test the formula using the flow rate and composition data of Part (a). (e) The system has been installed and made operational, and at some point the concentration of H2S in the feed stream suddenly changes. A sample of the blended gas is collected and analyzed a short time later and the mole ratio of H2S to SO2 is not the required 2:1. List as 3. A stream containing H2S and inert gases and a second stream of pure SO2 are fed to a sulfur recovery reactor, where the reaction 2H2S+SO2+3S+2H2O takes place. The feed rates are adjusted so that the ratio of H2S to SO2 in the combined feed is always stoichiometric In the normal operation of the reactor the flow rate and composition of the H2S feed stream both fluctuate. In the past, each time either variable changed the required SO2 feed rate had to be reset by adjusting a valve in the feed line. A control system has been installed to automate this process. The H2S feed stream passes through an electronic flowmeter that transmits a signal Rf directly proportional to the molar flow rate of the stream, nj. When nf=100 kmol/h, the transmitted signal Rf=15mV. The mole fraction of H2S in this stream is measured with a thermal conductivity detector, which transmits a signal Ra. Analyzer calibration data are as follows: The controller takes as input the transmitted values of R and Ra and calculates and transmits a voltage signal Rc to a flow control valve in the SO2 line, which opens and closes to an extent dependent on the value of Rc. A plot of the SO2 flow rate, nc, versus Rc on rectangular coordinates is a straight line through the points (Rc=10.0mV,nc=25.0kmol/h) and (Rc= 25.0mV;nc=60.0kmol/h). a. Why would it be important to feed the reactants in stoichiometric proportion? (Hint: SO2 and especially H2S are serious pollutants.) What are several likely reasons for wanting to automate the SO2 feed rate adjustment? (b) If the first stream contains 85.0 mole %H2S and enters the unit at a rate of (c) Fit a function to the H2S analyzer calibration data to derive an expression for x as a function of Ra. Check the fit by plotting both the function and the calibration data on the same graph. (d) Derive a formula for Rc from specified values of Rf and Ra, using the result of Part (c) in the derivation. (This formula would be built into the controller.) Test the formula using the flow rate and composition data of Part (a). (e) The system has been installed and made operational, and at some point the concentration of H2S in the feed stream suddenly changes. A sample of the blended gas is collected and analyzed a short time later and the mole ratio of H2S to SO2 is not the required 2:1. List as 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


