Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show all work The following chemical reaction O+ClOCl+O2 is studied when the [ClO]=2.34M, much greater than the [O]. When analyzing the data from this
Please show all work 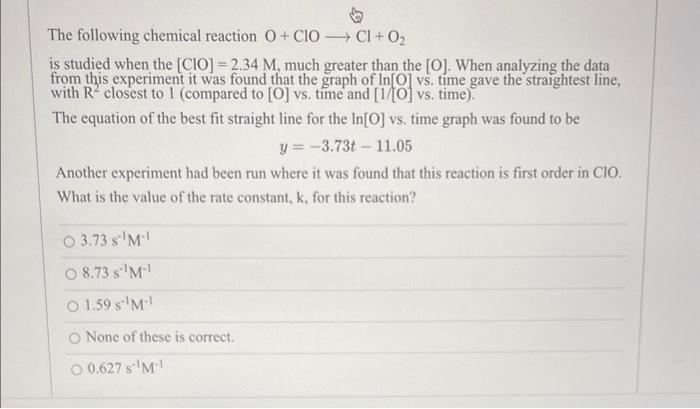
The following chemical reaction O+ClOCl+O2 is studied when the [ClO]=2.34M, much greater than the [O]. When analyzing the data from this experiment it was found that the graph of In [O] vs. time gave the straightest line, with R2 closest to 1 (compared to [O] vs. time and [1/[O] vs. time). The equation of the best fit straight line for the ln[O] vs. time graph was found to be y=3.73t11.05 Another experiment had been run where it was found that this reaction is first order in ClO. What is the value of the rate constant, k, for this reaction? 3.73s1M18.73s1M11.59s1M1 None of these is correct. 0.627s1M1 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


