Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) Making use of the fact that Eq. (6.20) is an exact differential expression, show that: (@Cp/aP)r = -T(a2v/aT2)p What is the result of
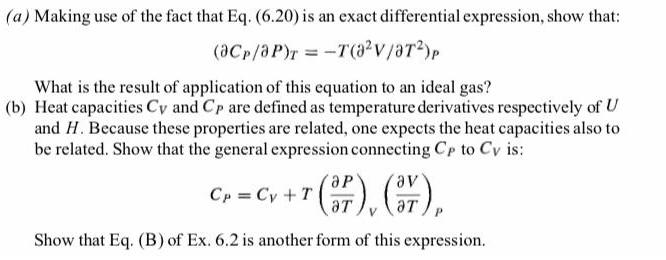
(a) Making use of the fact that Eq. (6.20) is an exact differential expression, show that: (@Cp/aP)r = -T(a2v/aT2)p What is the result of application of this equation to an ideal gas? (b) Heat capacities Cy and Cp are defined as temperature derivatives respectively of U and H. Because these properties are related, one expects the heat capacities also to be related. Show that the general expression connecting Cp to Cy is: C, - Cy +7 (), (), de, Cp = Cy +T aT Show that Eq. (B) of Ex. 6.2 is another form of this expression.
Step by Step Solution
★★★★★
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60d02c4542af8_219589.pdf
180 KBs PDF File
60d02c4542af8_219589.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


