Question
STARTING AMOUNT X ADD FACTOR x( ) 1 Convert the concentration of 0.700 M NaSO4 to g/mL 6.022 x 1023 M NaSO4 g/mL 0.0994
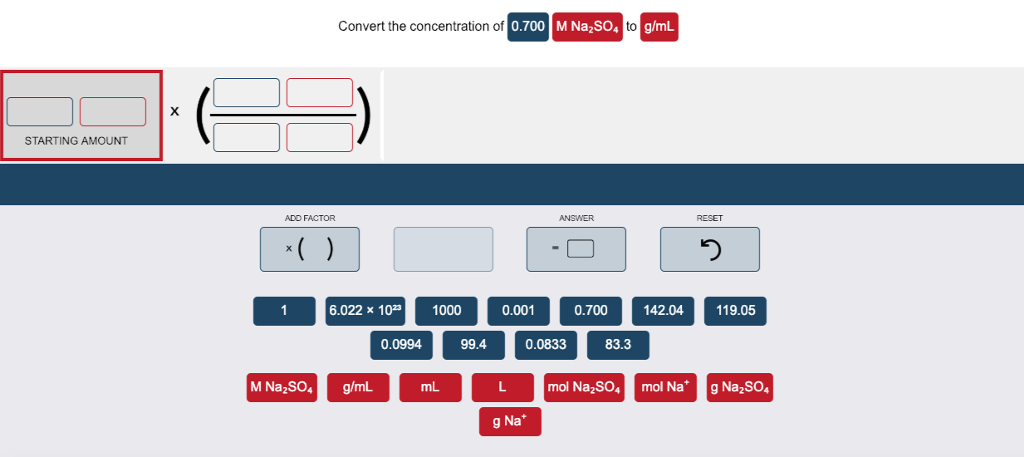
STARTING AMOUNT X ADD FACTOR x( ) 1 Convert the concentration of 0.700 M NaSO4 to g/mL 6.022 x 1023 M NaSO4 g/mL 0.0994 1000 mL 99.4 0.001 L ANSWER 0.0833 g Na* 0.700 83.3 mol NaSO4 142.04 RESET 3 119.05 mol Na* g NaSO4
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
07 M NaSO4 MEANS Of NaSO4 PRESENT MASS OF NaSO4 F 1 MOLES IN ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Database Systems Design Implementation and Management
Authors: Carlos Coronel, Steven Morris
13th edition
1337627909, 978-1337627900
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



