Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show how to work through this problem type so that I may apply it towards other questions similar to this. Thank you! When one
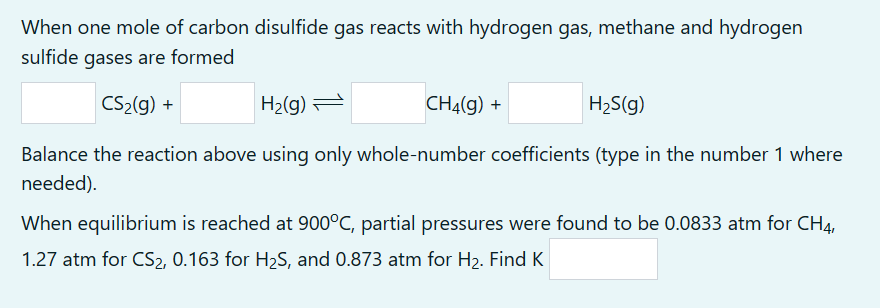
Please show how to work through this problem type so that I may apply it towards other questions similar to this. Thank you!
When one mole of carbon disulfide gas reacts with hydrogen gas, methane and hydrogen sulfide gases are formed CS2(g)+H2(g)CH4(g)+H2S(g) Balance the reaction above using only whole-number coefficients (type in the number 1 where needed). When equilibrium is reached at 900C, partial pressures were found to be 0.0833atm for CH4, 1.27 atm for CS2,0.163 for H2S, and 0.873 atm for H2. Find KStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


