Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show step by step solution to the answers provided on the right. a 2. An equilibrium separation process of a feed stream into vapor
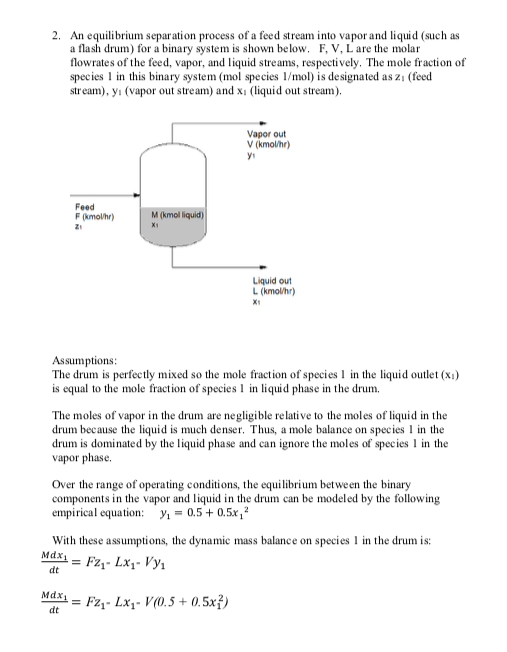

Please show step by step solution to the answers provided on the right.
a 2. An equilibrium separation process of a feed stream into vapor and liquid (such as a flash drum) for a binary system is shown below. F, V, L are the molar flowrates of the feed, vapor, and liquid streams, respectively. The mole fraction of species 1 in this binary system (mol species 1/mol) is designated as z (feed stream), y. (vapor out stream) and xi (liquid out stream), Vapor out V (kmothr) yi Feed Fmother) Z Mikmel liquid Liquid out L (kmol/hr) X1 Assumptions: The drum is perfectly mixed so the mole fraction of species 1 in the liquid outlet (xi) is equal to the mole fraction of species 1 in liquid phase in the drum. The moles of vapor in the drum are negligible relative to the moles of liquid in the drum because the liquid is much denser. Thus, a mole balance on species 1 in the drum is dominated by the liquid phase and can ignore the moles of species 1 in the vapor phase. Over the range of operating conditions, the equilibrium between the binary components in the vapor and liquid in the drum can be modeled by the following empirical equation: y = 0.5 +0.5x, ? With these assumptions, the dynamic mass balance on species 1 in the drum is: Mdx1 = F2 - LX - Vy dt Mdx = F2 - LX - V(0.5 +0.5x) dt a. Write the transfer function in standard form that describes the change in xi(t) if the inlet concentration, zi(t), changes. Over the range of operating conditions F, L, and V are constant (therefore M is also constant). X' (s) Z'(s) b. The process is running at steady state, with L - 40 kmol/min and V-60 kmol/min. At steady state, 21 -0.4, x1 -0.537 and y -0.194. M-800 kmol. What are the the gain and time constant of the transfer function in part a (with units)? K - 1.38 (no units) T-11,1 min c. From steady state, the inlet concentration of zi is reduced to 0.3. Use the transfer function to estimate the new steady state concentration of species 1 in the liquid phase (x1). X 0.399 a 2. An equilibrium separation process of a feed stream into vapor and liquid (such as a flash drum) for a binary system is shown below. F, V, L are the molar flowrates of the feed, vapor, and liquid streams, respectively. The mole fraction of species 1 in this binary system (mol species 1/mol) is designated as z (feed stream), y. (vapor out stream) and xi (liquid out stream), Vapor out V (kmothr) yi Feed Fmother) Z Mikmel liquid Liquid out L (kmol/hr) X1 Assumptions: The drum is perfectly mixed so the mole fraction of species 1 in the liquid outlet (xi) is equal to the mole fraction of species 1 in liquid phase in the drum. The moles of vapor in the drum are negligible relative to the moles of liquid in the drum because the liquid is much denser. Thus, a mole balance on species 1 in the drum is dominated by the liquid phase and can ignore the moles of species 1 in the vapor phase. Over the range of operating conditions, the equilibrium between the binary components in the vapor and liquid in the drum can be modeled by the following empirical equation: y = 0.5 +0.5x, ? With these assumptions, the dynamic mass balance on species 1 in the drum is: Mdx1 = F2 - LX - Vy dt Mdx = F2 - LX - V(0.5 +0.5x) dt a. Write the transfer function in standard form that describes the change in xi(t) if the inlet concentration, zi(t), changes. Over the range of operating conditions F, L, and V are constant (therefore M is also constant). X' (s) Z'(s) b. The process is running at steady state, with L - 40 kmol/min and V-60 kmol/min. At steady state, 21 -0.4, x1 -0.537 and y -0.194. M-800 kmol. What are the the gain and time constant of the transfer function in part a (with units)? K - 1.38 (no units) T-11,1 min c. From steady state, the inlet concentration of zi is reduced to 0.3. Use the transfer function to estimate the new steady state concentration of species 1 in the liquid phase (x1). X 0.399Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


