Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show steps for the energy involved missed and the lattice energy of CaCl2. Thank you The heat of formation of CaCl2 is 795kJ/mol. The
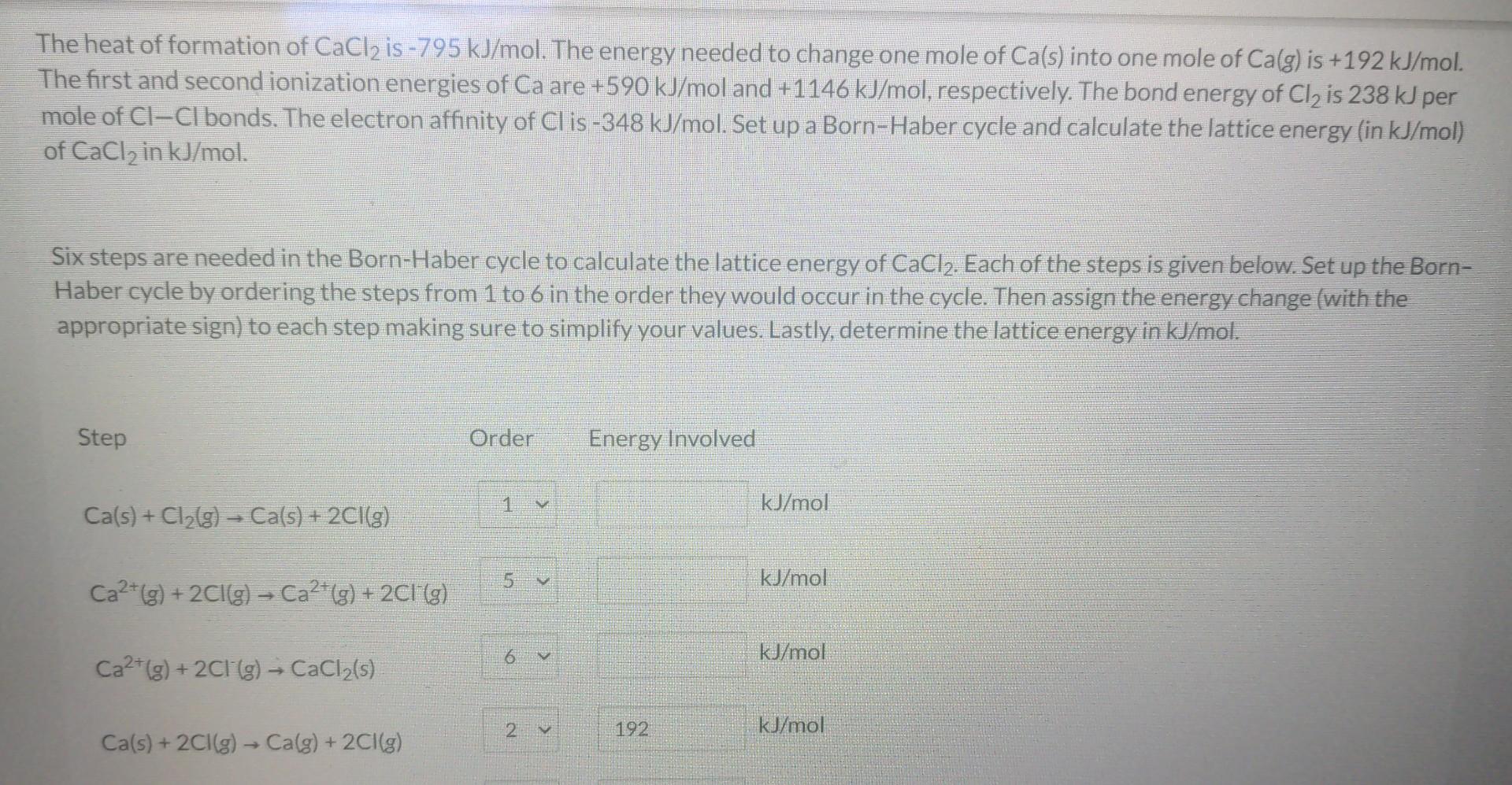
please show steps for the energy involved missed and the lattice energy of CaCl2. Thank you
The heat of formation of CaCl2 is 795kJ/mol. The energy needed to change one mole of Ca(s) into one mole of Ca(g) is +192kJ/mol. The first and second ionization energies of Ca are +590kJ/mol and +1146kJ/mol, respectively. The bond energy of Cl2 is 238kJ per mole of ClCl bonds. The electron affinity of Cl is 348kJ/mol. Set up a Born-Haber cycle and calculate the lattice energy (in kJ/mol ) of CaCl2 in kJ/mol. Six steps are needed in the Born-Haber cycle to calculate the lattice energy of CaCl2. Each of the steps is given below. Set up the BornHaber cycle by ordering the steps from 1 to 6 in the order they would occur in the cycle. Then assign the energy change (with the appropriate sign) to each step making sure to simplify your values. Lastly, determine the lattice energy in kJ/molStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


