Question
1. A vessel, divided into two parts by a partition, contains 5 mol of nitrogen gas at 75C and 30 bar on one side
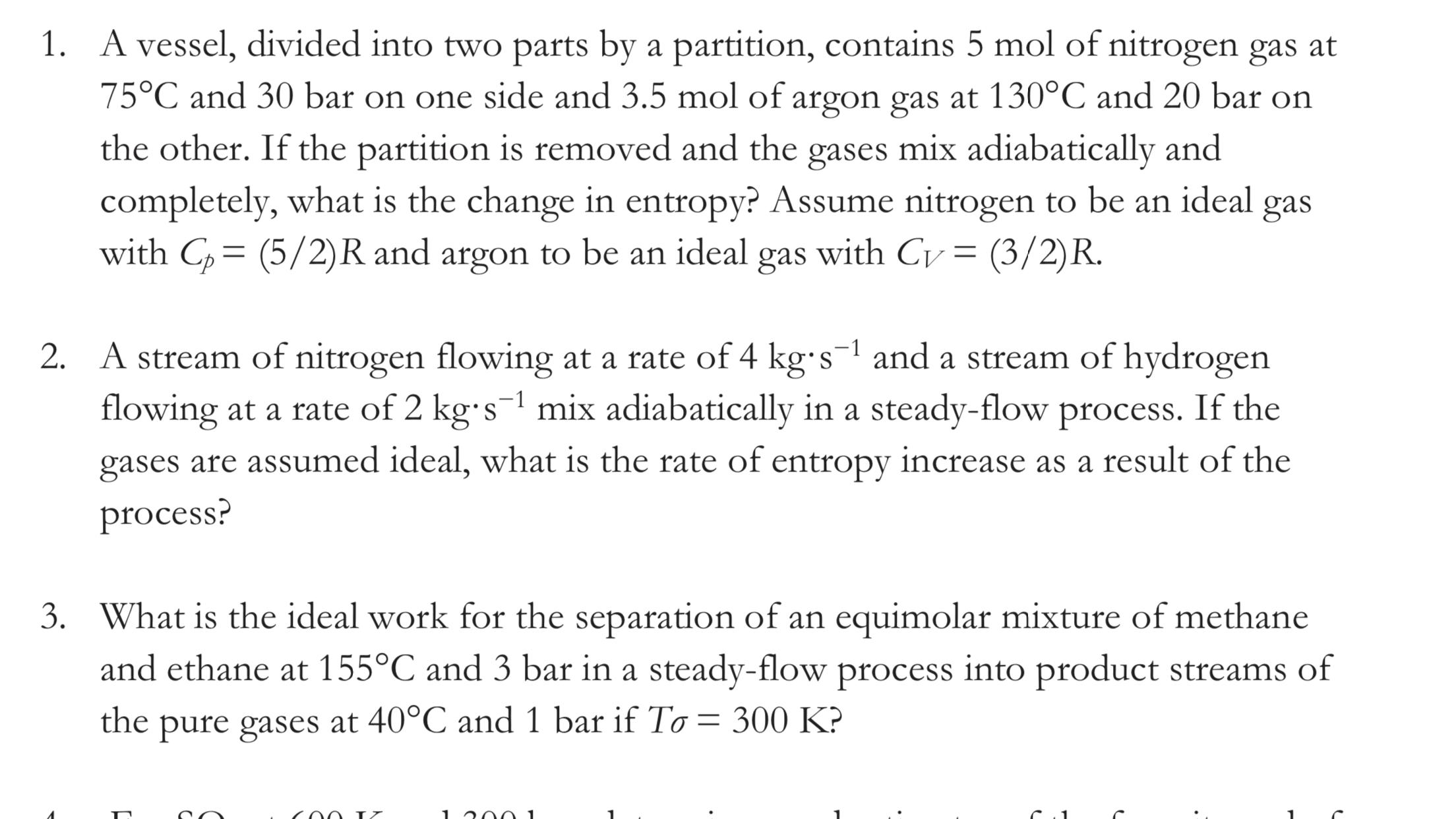
1. A vessel, divided into two parts by a partition, contains 5 mol of nitrogen gas at 75C and 30 bar on one side and 3.5 mol of argon gas at 130C and 20 bar on the other. If the partition is removed and the gases mix adiabatically and completely, what is the change in entropy? Assume nitrogen to be an ideal gas with Cp = (5/2)R and argon to be an ideal gas with Cv = (3/2)R. 2. A stream of nitrogen flowing at a rate of 4 kg-s and a stream of hydrogen flowing at a rate of 2 kgs mix adiabatically in a steady-flow process. If the gases are assumed ideal, what is the rate of entropy increase as a result of the process? 3. What is the ideal work for the separation of an equimolar mixture of methane and ethane at 155C and 3 bar in a steady-flow process into product streams of the pure gases at 40C and 1 bar if To = 300 K? 100 IZ 1.2001 6.1
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



