Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show the process to get to the given answer. Reaction of aluminum sulfate with alkalinity: Al2(SO4)314.3H2O+6HCO32Al(OH)33H2O+6CO2+8H2O+3SO42600g/mol366g/mol Reaction of aluminum sulfate with lime [Ca(OH)2] :
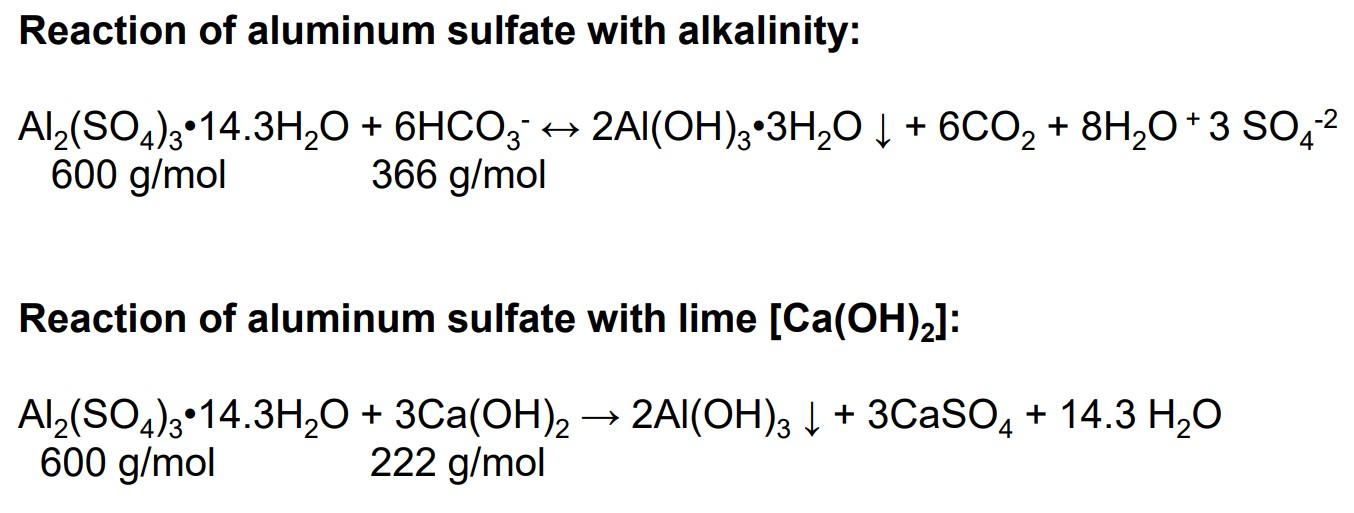
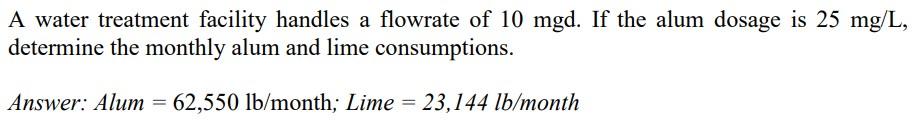
Please show the process to get to the given answer.
Reaction of aluminum sulfate with alkalinity: Al2(SO4)314.3H2O+6HCO32Al(OH)33H2O+6CO2+8H2O+3SO42600g/mol366g/mol Reaction of aluminum sulfate with lime [Ca(OH)2] : Al2(SO4)314.3H2O+3Ca(OH)22Al(OH)3+3CaSO4+14.3H2O600g/mol222g/mol A water treatment facility handles a flowrate of 10mgd. If the alum dosage is 25mg/L, determine the monthly alum and lime consumptions. Answer: Alum =62,550lb/month; Lime =23,144lb/monthStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


