Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show work so that I may take the math and apply it to other questions of this type. Consider the system SO3(g)SO2(g)+21O2(g) For how
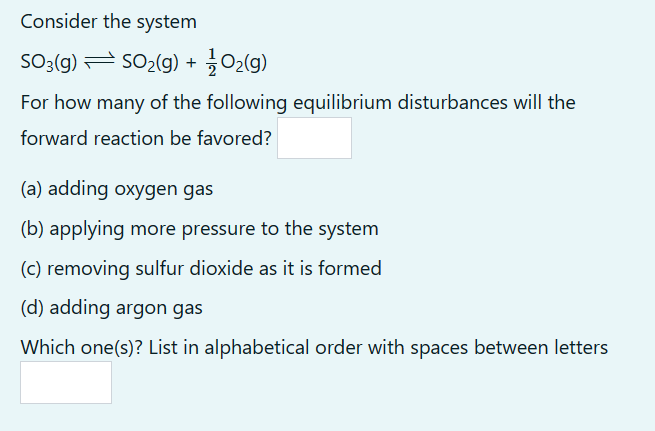
Please show work so that I may take the math and apply it to other questions of this type.
Consider the system SO3(g)SO2(g)+21O2(g) For how many of the following equilibrium disturbances will the forward reaction be favored? (a) adding oxygen gas (b) applying more pressure to the system (c) removing sulfur dioxide as it is formed (d) adding argon gas Which one(s)? List in alphabetical order with spaces between lettersStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


