Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve 1 a and b 1. ) The half-life period for a certain first order reaction is 45.5 minutes. How long will it take
Please solve 1 a and b 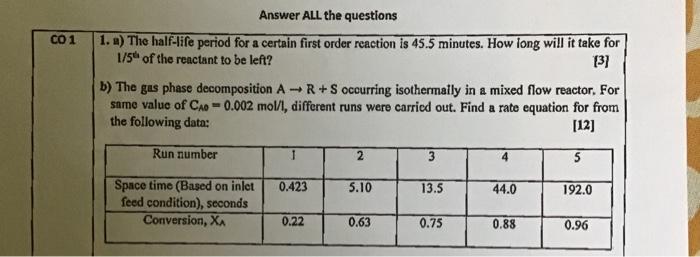
1. ) The half-life period for a certain first order reaction is 45.5 minutes. How long will it take for 1/5t of the reactant to be left? [3] b) The gas phase decomposition AR+S occurring isothermally in a mixed flow reactor. For same value of CAO=0.002mol/l, different runs were carricd out. Find a rate equation for from the following data: [12] 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


