Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve all of them 1. ( 30 pts) 4.5 moles of an ideal gas are compressed from 1.0atm to 3.0atm at a constant 273K
Please solve all of them 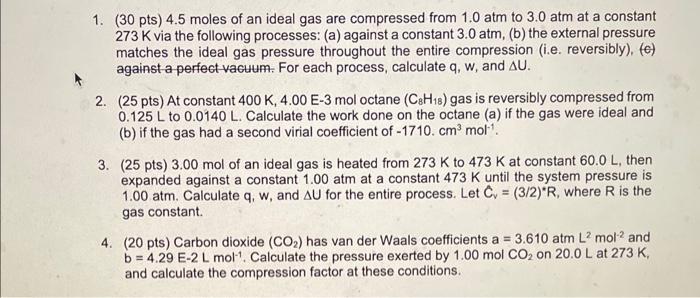
1. ( 30 pts) 4.5 moles of an ideal gas are compressed from 1.0atm to 3.0atm at a constant 273K via the following processes: (a) against a constant 3.0atm, (b) the external pressure matches the ideal gas pressure throughout the entire compression (i.e. reversibly), (c) against a perfect vacuum. For each process, calculate q,w, and U. 2. ( 25pts) At constant 400K,4.00E3mol octane (C8H18) gas is reversibly compressed from 0.125L to 0.0140L. Calculate the work done on the octane (a) if the gas were ideal and (b) if the gas had a second virial coefficient of 1710.cm3mol1. 3. (25 pts) 3.00mol of an ideal gas is heated from 273K to 473K at constant 60.0L, then expanded against a constant 1.00atm at a constant 473K until the system pressure is 1.00atm. Calculate q,w, and U for the entire process. Let Cv=(3/2)R, where R is the gas constant. 4. (20 pts) Carbon dioxide (CO2) has van der Waals coefficients a =3.610atmL2mol2 and b=4.29E2Lmol1. Calculate the pressure exerted by 1.00molCO2 on 20.0Lat273K, and calculate the compression factor at these conditions 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


