Answered step by step
Verified Expert Solution
Question
1 Approved Answer
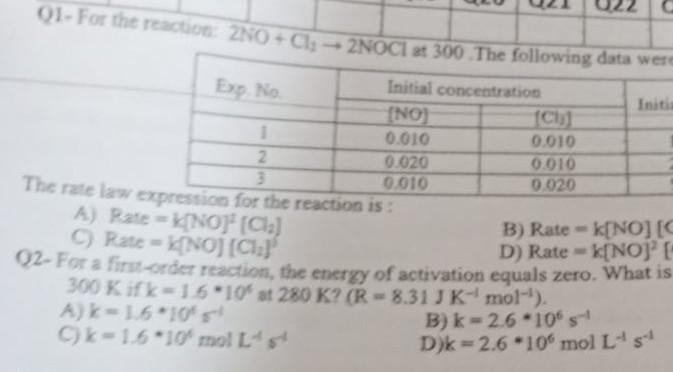
Please solve all question quickly 01- For the reaction: ZNO + Cl2NOCI at 300 The following data were Exp. No Initial concentration Initi (NO) CI)
Please solve all question quickly
01- For the reaction: ZNO + Cl2NOCI at 300 The following data were Exp. No Initial concentration Initi (NO) CI) 1 0.010 0.010 2 0.020 0.010 3 0.010 0.020 The rate law expression for the reaction is: A) Rate=NO" (Cla) B) Ratek[NO] [S C C) Rate = NOJ (012) D) Rate -k[NOJE 92- For a first-orcz reaction, the energy of activation equals zero. What is 300 Kifk-16*10 st 280 K? (R-8.31 JK-mol-'). Ak-1.610 B) k-2.6 -10% Ck-1.6 10' molts D) -2.6 -10% mol LsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


