Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve and explain!! thank youu 1. a. Chlorobenzene undergoes electrophilic aromatic substitution with Bry/FeBrz to form the products below. (5 pts) Bre, FeBrg Br
please solve and explain!! thank youu 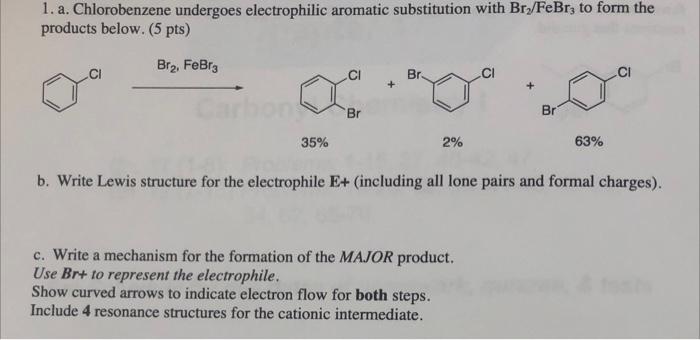
1. a. Chlorobenzene undergoes electrophilic aromatic substitution with Bry/FeBrz to form the products below. (5 pts) Bre, FeBrg Br + Br Br 35% 2% 63% b. Write Lewis structure for the electrophile E+ (including all lone pairs and formal charges). a c. Write a mechanism for the formation of the MAJOR product. Use Br+ to represent the electrophile. Show curved arrows to indicate electron flow for both steps. Include 4 resonance structures for the cationic intermediate 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


