Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve and match When determining the equilibrium concentrations for the reaction AB(g)A(g)+B(g)Kc=[AB][A][B] a simplifying assumption can be used under certain conditions to avoid solving
please solve and match 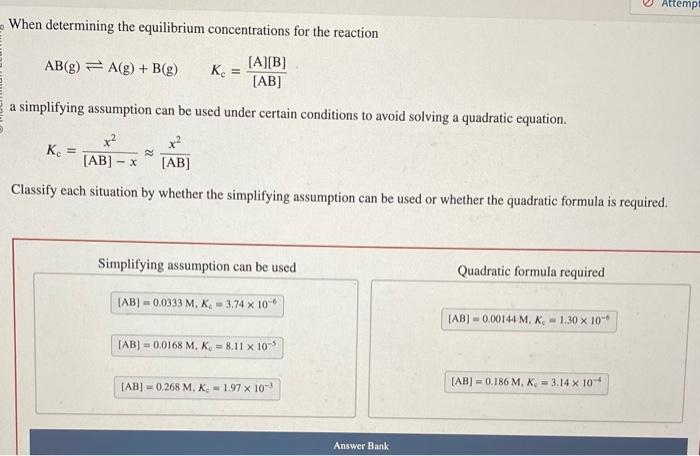
When determining the equilibrium concentrations for the reaction AB(g)A(g)+B(g)Kc=[AB][A][B] a simplifying assumption can be used under certain conditions to avoid solving a quadratic equation. Kc=[AB]xx2[AB]x2 Classify each situation by whether the simplifying assumption can be used or whether the quadratic formula is required. Simplifying assumption can be used Quadratic formula required [AB]=0.0333M,KC=3.74106 [AB]=0.00144M,Kc=1.30106 [AB]=0.0168M,K0=8.11105 [AB]=0.268M,Ke=1.97103 [AB]=0.186M,Ke=3.14104 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


