Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve and write code in Matlab only solve for OXYGEN also, please rewrite eqn. 1 for rootfinding on paper. thank you! Project Introduction and
please solve and write code in Matlab 

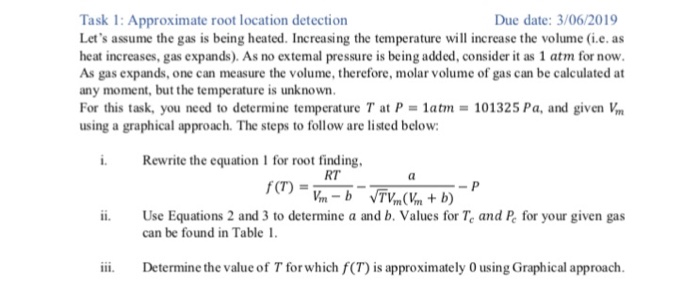
only solve for OXYGEN
also, please rewrite eqn. 1 for rootfinding on paper. thank you!
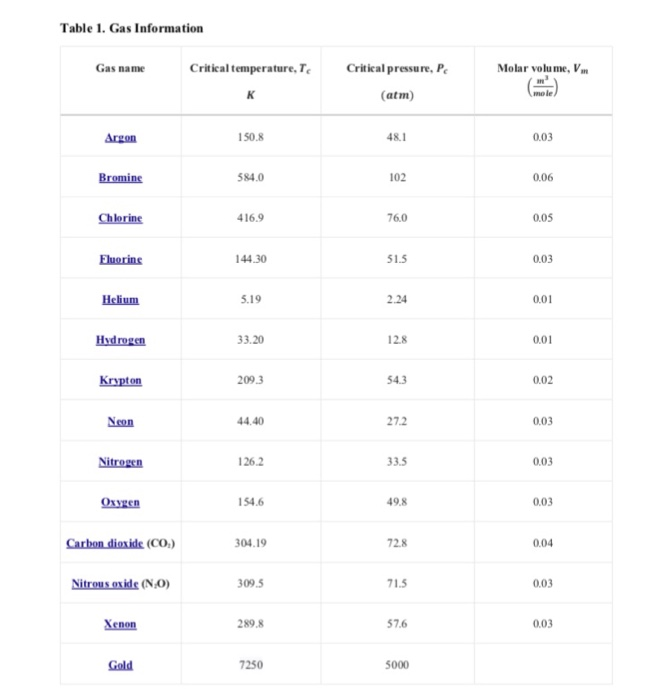
Project Introduction and Theory The ideal gas equation has been widely used to relate Temperature, Pressure and Volume of Gases. The equation can be written as. RT rr2 Where, wn = Molar volume of gas, R Gas constan, T = Temperature, and P Gas pressure There are several other equations that characterize gas better than the Ideal Gas equation, like the van der Waals equation. This equation, however, is not as accurate for temperatures above the critical temperature, so equations like the Redlich-Kwong (RK) equation of state would generally be used at higher temperatures. The RK equation is shown below RT Where R2T 25 0.42748 RTe 0.08664-e b In Equations 2 and 3, Te is the critical temperature and Pe is the critical pressre. And, R 8.314 Kmol Task 1: Approximate root location detection Let's assume the gas is being heated. Increasing the temperature will increase the volume (i.e. as heat increases, gas expands). As no extemal pressure is being added, consider it as 1 atm for now. As gas expands, one can measure the volume, therefore, molar volume of gas can be calculated at any moment, but the temperature is unknown For this task, you need to determine temperature T at P-1atm 101325 Pa, and given V using a graphical approach. The steps to follow are listed below Due date: 3/06/2019 i. Rewrite the equation 1 for root finding ii. Use Equations 2 and 3 to determine a and b. Values for Te and Pe for your given gas i Determine the value of T for which f(T) is approximately O using Graphical approach RT can be found in Table 1 Table 1. Gas Information Critical temperature,T Critical pressure, P Molar volume, Vm Gas name (atm) mole Argon 150.8 48.1 0.03 584.0 102 0.06 416.9 76.0 0.05 44.30 51.5 0.03 Helium 5.19 2.24 0.01 33.20 12.8 0.01 209.3 54.3 0.02 Neon 44.40 27.2 0.03 126.2 33.5 0.03 Oxygen 154.6 49.8 0.03 04.19 72.8 0.04 Nitrous oxide (NO) 09.5 71.5 003 Xenon 289.8 57.6 0.03 Gold 7250 5000 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


