Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE SOLVE IT ASAP USING MATLAB WRITE THE CODES Question 3-Consider the reaction between mesitylene (M) and hydrogen (H) over a Houdry Detrol catalyst. The
PLEASE SOLVE IT ASAP USING MATLAB WRITE THE CODES 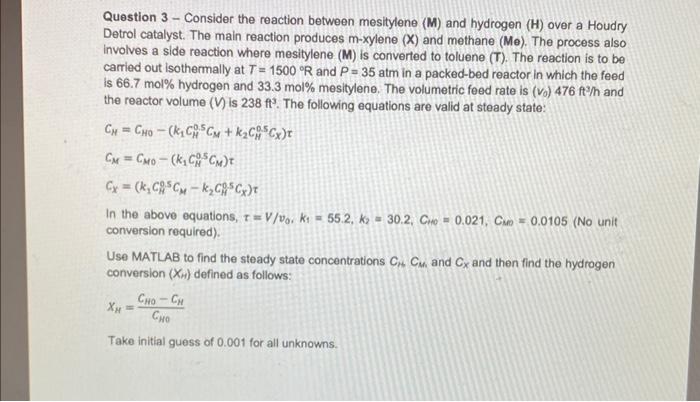
Question 3-Consider the reaction between mesitylene (M) and hydrogen (H) over a Houdry Detrol catalyst. The main reaction produces mxylene(X) and methane (Me). The process also involves a side reaction where mesitylene (M) is converted to toluene (T). The reaction is to be carried out isothermally at T=1500R and P=35 atm in a packed-bed reactor in which the feed is 66.7mol% hydrogen and 33.3mol% mesitylene. The volumetric feed rate is (v0)476ft3/h and the reactor volume (V) is 238ft3. The following equations are valid at steady state: CH=CH0(k1CH0.5CM+k2CH05CX)CM=CM0(k1CH0.5CM)CX=(k1CH0.5CMk2CH05CX) In the above equations, =V/v0,k1=55.2,k2=30.2,C10=0.021,CM0=0.0105 (No unit conversion required). Use MATLAB to find the steady state concentrations CMCM and CX and then find the hydrogen conversion (XH) defined as follows: H=CH0CH0CH Take initial guess of 0.001 for all unknowns 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


