Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve this chemical engineering question (1) 1. Pig iron is produced in a blast furnace by charging the ore, flux and coke, of which
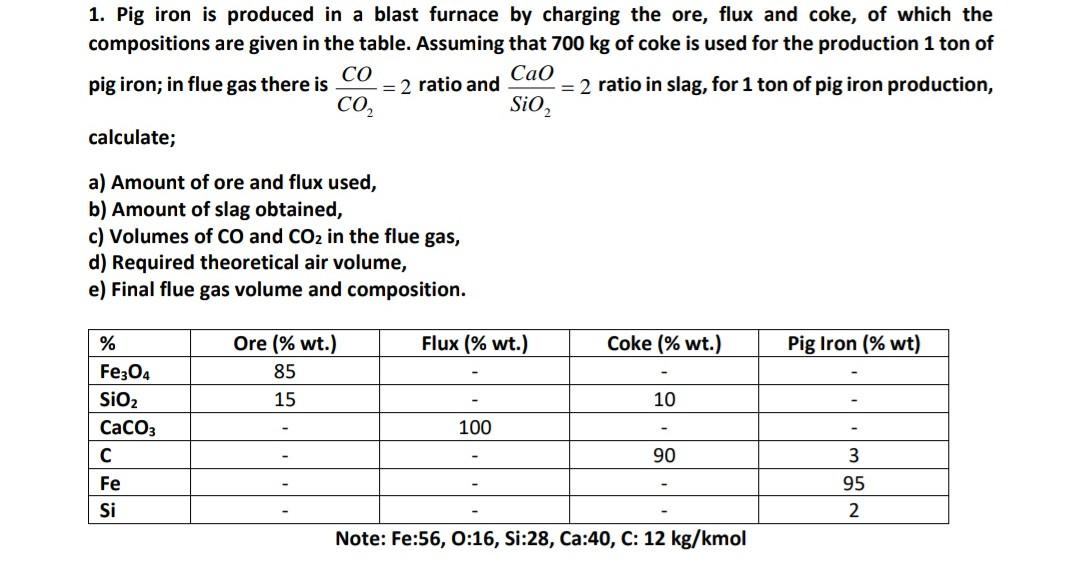
please solve this chemical engineering question (1)
1. Pig iron is produced in a blast furnace by charging the ore, flux and coke, of which the compositions are given in the table. Assuming that 700kg of coke is used for the production 1 to of pig iron; in flue gas there is CO2CO=2 ratio and SiO2CaO=2 ratio in slag, for 1 ton of pig iron production, calculate; a) Amount of ore and flux used, b) Amount of slag obtained, c) Volumes of CO and CO2 in the flue gas, d) Required theoretical air volume, e) Final flue gas volume and composition. Note: Fe:56, 0:16, Si:28, Ca:40, C: 12kg/kmolStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


