Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve this kinetic problem At elevated temperatures, helium dissolves in Ni predominantly as He atoms trapped in Ni vacancies (Hev complexes substitutional He atoms),
Please solve this kinetic problem
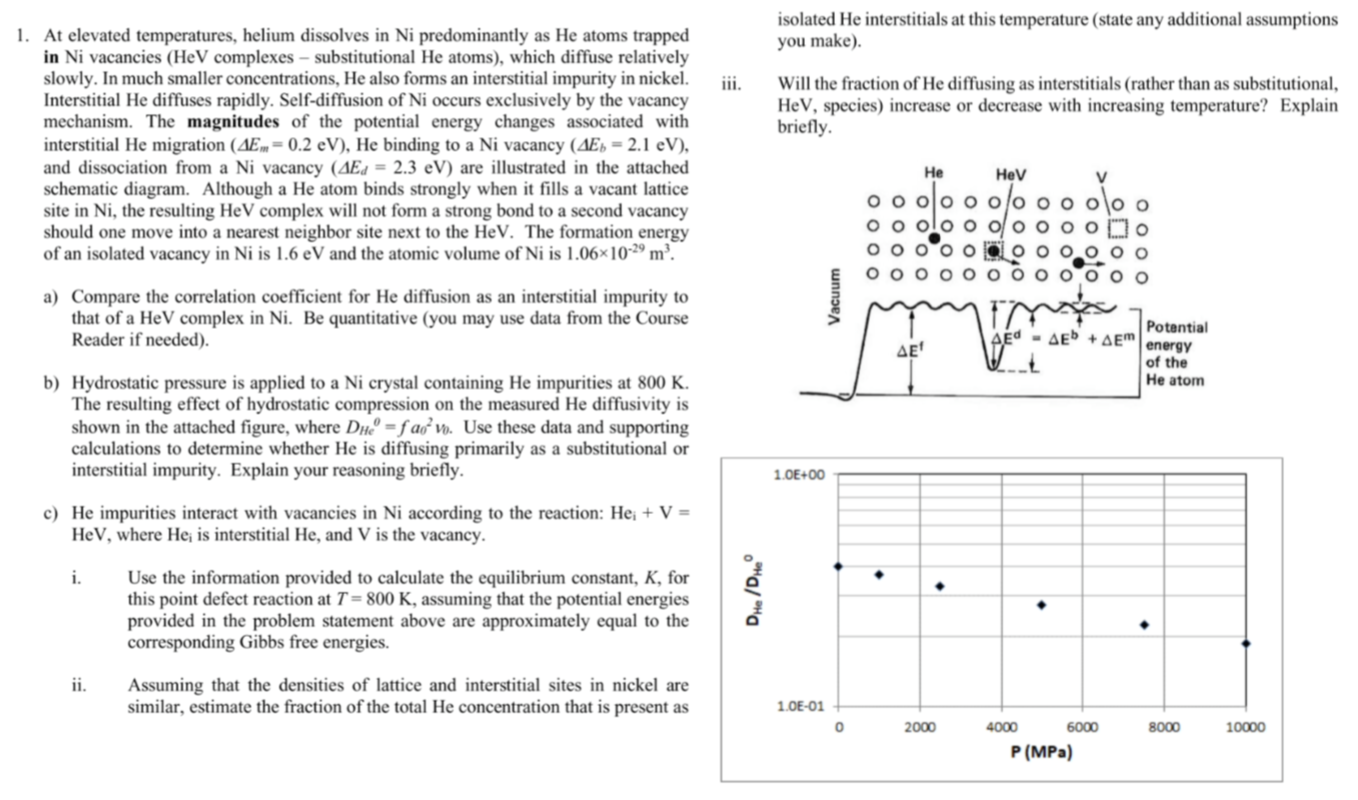
At elevated temperatures, helium dissolves in Ni predominantly as He atoms trapped in Ni vacancies (Hev complexes substitutional He atoms), which diffuse relatively slowly. In much smaller concentrations. He also forms an interstitial impurity in nickel. Interstitial He diffuses rapidly. Self-diffusion of Ni occurs exclusively by the vacancy mechanism. The magnitudes of the potential energy changes associated with interstitial He migration ( 0.2 eV). He binding to a Ni vacancy (LIEB 2.1 eV), and dissociation from a Ni vacancy = 2.3 eV) are illustrated in the attached schematic diagram. Although a He atom binds strongly when it fills a vacant lattice site in Ni, the resulting Hev complex will not form a strong bond to a second vacancy should one move into a nearest neighbor site next to the HeV. The formation energy of an isolated vacancy in Ni is 1.6 eV and the atomic volume of Ni is ne. a) Compare the correlation coemcient for He diffusion as an interstitial impurity to that of a Hev complex in Ni. Be quantitative (you may use data from the Course Reader if needed). b) Hydrostatic pressure is applied to a Ni crystal containing He impurities at 800 K. The resulting effect of hydrostatic compression on the measured He diffusivity is shown in the attached figure, where fao:vo. Use these data and supporting calculations to determine whether He is diffusing primarily as a substitutional or interstitial impurity. Explain your reasoning briefly. c) He impurities interact with vacancies in Ni according to the reaction: Hei + V = HeV, where Hei is interstitial He, and V is the vacancy. iii. isolated He interstitials at this temperature (state any additional assumptions you make). Will the fraction of He diffusing as interstitials (rather than as substitutional. HeV, species) increase or decrease with increasing temperature? Explain briefly. o o o o i. ii. Use the information provided to calculate the equilibrium constant, K, for this point defect reaction at T 800 K. assuming that the potential energies provided in the problem statement above are approximately equal to the corresponding Gibbs free energies. Assuming that the densities of lattice and interstitial sites in nickel are similar, estimate the fraction of the total He concentration that is present as He 00 ooo o o o 0 0 00 0 o d AEb P (MP.) 0 00 o Clo 0 00 00 0 of the He atom zomo
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


