Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve this problem and explain how did you do it P3. (8 pts) A 50:50 mol% MeOH/EtOH mixture is supplied to an adiabatic flash
please solve this problem and explain how did you do it 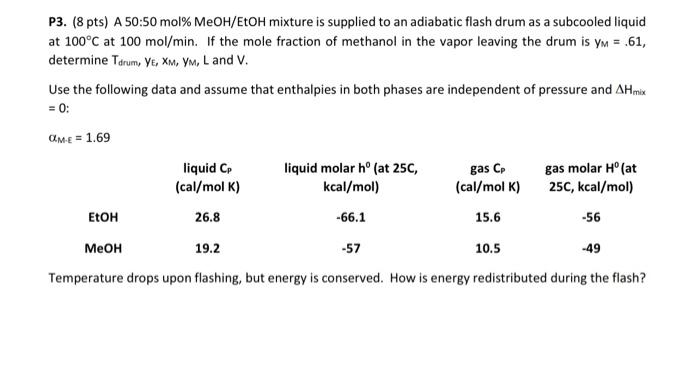
P3. (8 pts) A 50:50 mol% MeOH/EtOH mixture is supplied to an adiabatic flash drum as a subcooled liquid at 100C at 100 mol/min. If the mole fraction of methanol in the vapor leaving the drum is ym = .61, determine Tarum, YE, XM, YM, Land V. Use the following data and assume that enthalpies in both phases are independent of pressure and AHmix = 0: CME = 1.69 liquid CP (cal/mol K) liquid molar h(at 25C, kcal/mol) gas CP (cal/mol K) gas molar H (at 25C, kcal/mol) -56 EtOH 26.8 -66.1 15.6 19.2 -57 10.5 -49 Temperature drops upon flashing, but energy is conserved. How is energy redistributed during the flash 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


