Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve this problem ASAP 2. Pressurized hydrogen gas is stored at 358 K in a 4.8 m outer diameter spherical container make of nickel.
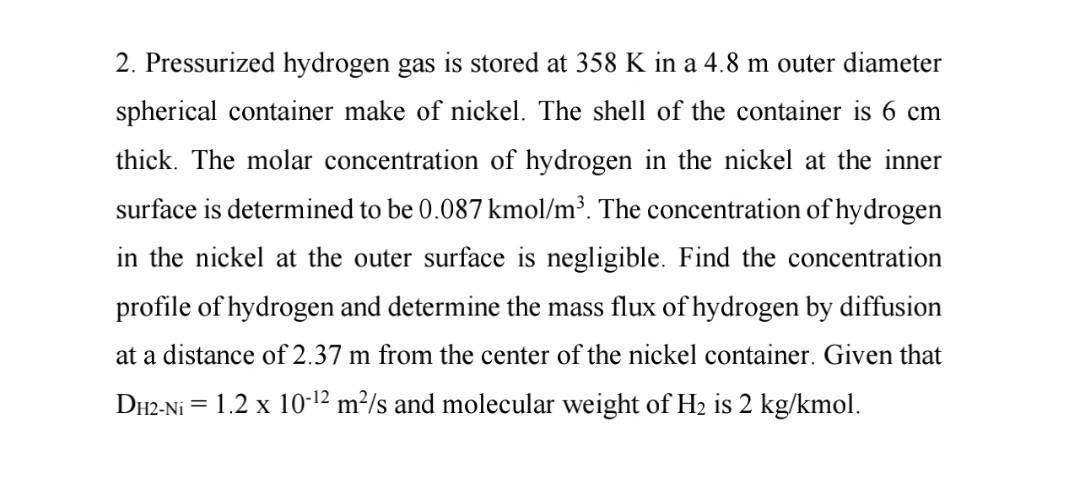
Please solve this problem ASAP
2. Pressurized hydrogen gas is stored at 358 K in a 4.8 m outer diameter spherical container make of nickel. The shell of the container is 6 cm thick. The molar concentration of hydrogen in the nickel at the inner surface is determined to be 0.087 kmol/m. The concentration of hydrogen in the nickel at the outer surface is negligible. Find the concentration profile of hydrogen and determine the mass flux of hydrogen by diffusion at a distance of 2.37 m from the center of the nickel container. Given that DH2-Ni = 1.2 x 10-12 m/s and molecular weight of H2 is 2 kg/kmolStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


