Answered step by step
Verified Expert Solution
Question
1 Approved Answer
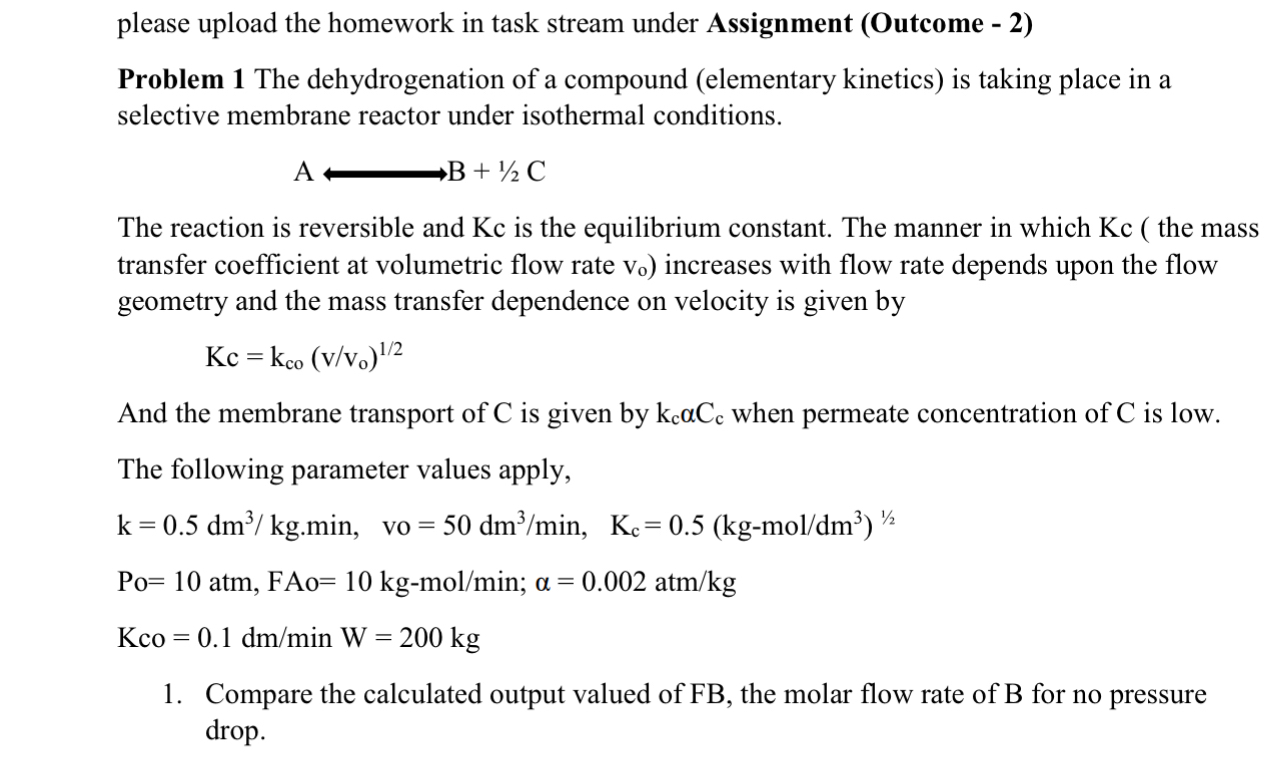
please upload the homework in task stream under Assignment ( Outcome - 2 ) Problem 1 The dehydrogenation of a compound ( elementary kinetics )
please upload the homework in task stream under Assignment Outcome
Problem The dehydrogenation of a compound elementary kinetics is taking place in a selective membrane reactor under isothermal conditions.
AlongrightarrowB
The reaction is reversible and is the equilibrium constant. The manner in which the mass transfer coefficient at volumetric flow rate increases with flow rate depends upon the flow geometry and the mass transfer dependence on velocity is given by
And the membrane transport of is given by when permeate concentration of is low.
The following parameter values apply,
atm,FAo;
KcoinW
Compare the calculated output valued of FB the molar flow rate of B for no pressure drop.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


