Please use EES. And answer all questions (no spam)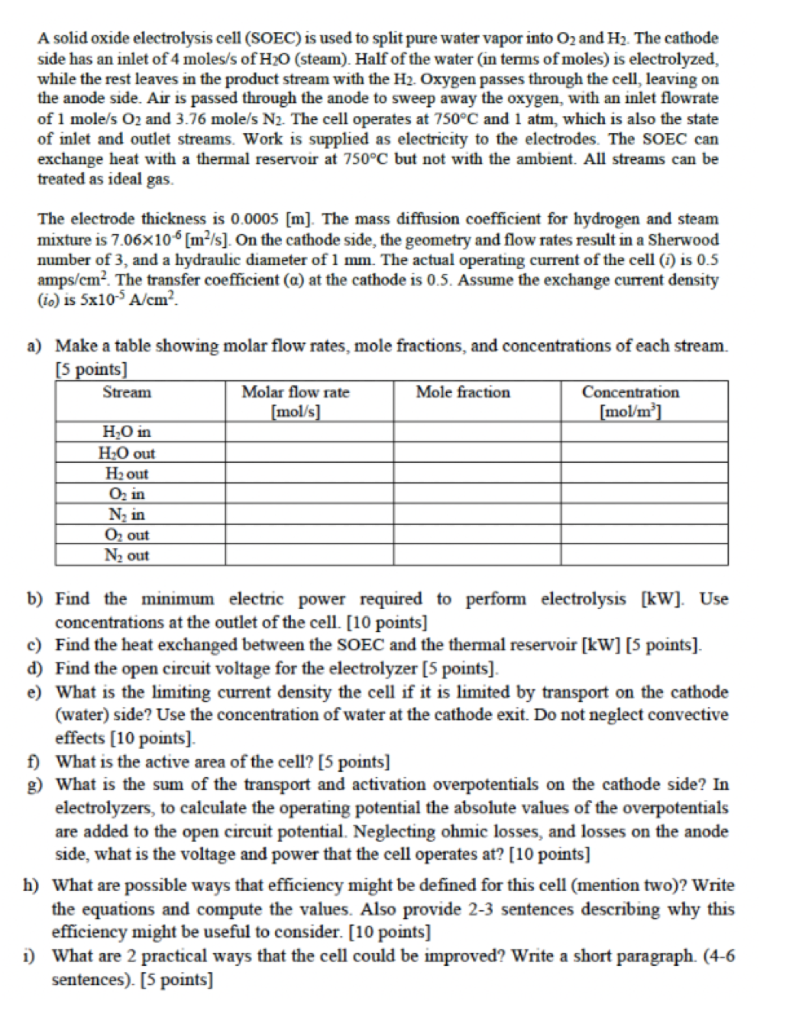
A solid oxide electrolysis cell (SOEC) is used to split pure water vapor into O2 and H2. The cathode side has an inlet of 4 moles/s of H20 (steam). Half of the water (in terms of moles) is electrolyzed, while the rest leaves in the product stream with the H2. Oxygen passes through the cell, leaving on the anode side. Air is passed through the anode to sweep away the oxygen, with an inlet flowrate of 1 mole/s O2 and 3.76 mole/s N2. The cell operates at 750C and 1 atm, which is also the state of inlet and outlet streams. Work is supplied as electricity to the electrodes. The SOEC can exchange heat with a thermal reservoir at 750C but not with the ambient. All streams can be treated as ideal gas. The electrode thickness is 0.0005 [m]. The mass diffusion coefficient for hydrogen and steam mixture is 7.06x10 [m/s]. On the cathode side, the geometry and flow rates result in a Sherwood number of 3, and a hydraulic diameter of 1 mm. The actual operating current of the cell (1) is 0.5 amps/cm. The transfer coefficient (a) at the cathode is 0.5. Assume the exchange current density (ic) is 5x10-A/cm? a) Make a table showing molar flow rates, mole fractions, and concentrations of each stream. [5 points) Stream Molar flow rate Mole fraction Concentration [mol/s) [mol/m] H0 in H:0 out H2 out O, in N, in O, out N2 out b) Find the minimum electric power required to perform electrolysis [kW]. Use concentrations at the outlet of the cell. [10 points] c) Find the heat exchanged between the SOEC and the thermal reservoir [kW] [5 points). d) Find the open circuit voltage for the electrolyzer (5 points) e) What is the limiting current density the cell if it is limited by transport on the cathode (water) side? Use the concentration of water at the cathode exit. Do not neglect convective effects (10 points) f) What is the active area of the cell? [5 points) g) What is the sum of the transport and activation overpotentials on the cathode side? In electrolyzers, to calculate the operating potential the absolute values of the overpotentials are added to the open circuit potential. Neglecting ohmic losses, and losses on the anode side, what is the voltage and power that the cell operates at? [10 points) h) What are possible ways that efficiency might be defined for this cell (mention two)? Write the equations and compute the values. Also provide 2-3 sentences describing why this efficiency might be useful to consider. [10 points] 1) What are 2 practical ways that the cell could be improved? Write a short paragraph. (4-6 sentences). [5 points)







