Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please use MatLab to solve KT +(BRT + (BT - A - The Benedict-Webb-Rubin equation of state is a version of the gas law for

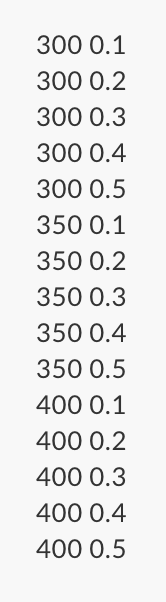
Please use MatLab to solve
KT +(BRT + (BT - A - The Benedict-Webb-Rubin equation of state is a version of the gas law for non-ideal situations. RT 1 (bRT-a) a a P= + + + 12) 52 03 76 2 where R = 8.314 kmo! is the universal gas constant. The constants A, B.C. a,b,c,a,and y are substance dependent, 7 = vM is the molar specific volume in where M is the molar kg mass in komol kimo! Write a program in a script file, to calculate the pressure of CO2 using the Benedict-Webb-Rubin equation for the volumes and temperature provided in the Tv.txt file (download from URlearns) and using the constants below. Table P3: Benedict-Webb-Rubin constants for pressure in kPa, specific volume in and temperature in K. kmol' Substance b B C u Y CO2 0.1386 2.7737 0.00721 0.04991 1.512 x 10* 1.404 x 105 8.47 x 10-5 0.00539 a Molar mass (M) for CO2 is 44 kg kmol Your program must import the data in from the Tv.txt file and assign the first column to a variable named T and the second column to a variable named v. Calculations for must be stored in a variable named v_bar and your final calculation for pressure must be stored in a variable named P. Using a single fprintf function, display the output in the format. All numbers must be printed with two digits past the decimal place. For CO2 at T = xx.xx K and v = xx.xx m^3/kg, p = xx.xx kPa. For co2 at T = xx.xx K and v = xx.xx m^3/kg, p = xx.xx kPa. For co2 at T = xx.xx K and v = xx.xx m^3/kg, p = xx.xx kPa. KT +(BRT + (BT - A - The Benedict-Webb-Rubin equation of state is a version of the gas law for non-ideal situations. RT 1 (bRT-a) a a P= + + + 12) 52 03 76 2 where R = 8.314 kmo! is the universal gas constant. The constants A, B.C. a,b,c,a,and y are substance dependent, 7 = vM is the molar specific volume in where M is the molar kg mass in komol kimo! Write a program in a script file, to calculate the pressure of CO2 using the Benedict-Webb-Rubin equation for the volumes and temperature provided in the Tv.txt file (download from URlearns) and using the constants below. Table P3: Benedict-Webb-Rubin constants for pressure in kPa, specific volume in and temperature in K. kmol' Substance b B C u Y CO2 0.1386 2.7737 0.00721 0.04991 1.512 x 10* 1.404 x 105 8.47 x 10-5 0.00539 a Molar mass (M) for CO2 is 44 kg kmol Your program must import the data in from the Tv.txt file and assign the first column to a variable named T and the second column to a variable named v. Calculations for must be stored in a variable named v_bar and your final calculation for pressure must be stored in a variable named P. Using a single fprintf function, display the output in the format. All numbers must be printed with two digits past the decimal place. For CO2 at T = xx.xx K and v = xx.xx m^3/kg, p = xx.xx kPa. For co2 at T = xx.xx K and v = xx.xx m^3/kg, p = xx.xx kPa. For co2 at T = xx.xx K and v = xx.xx m^3/kg, p = xx.xx kPaStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


