Question
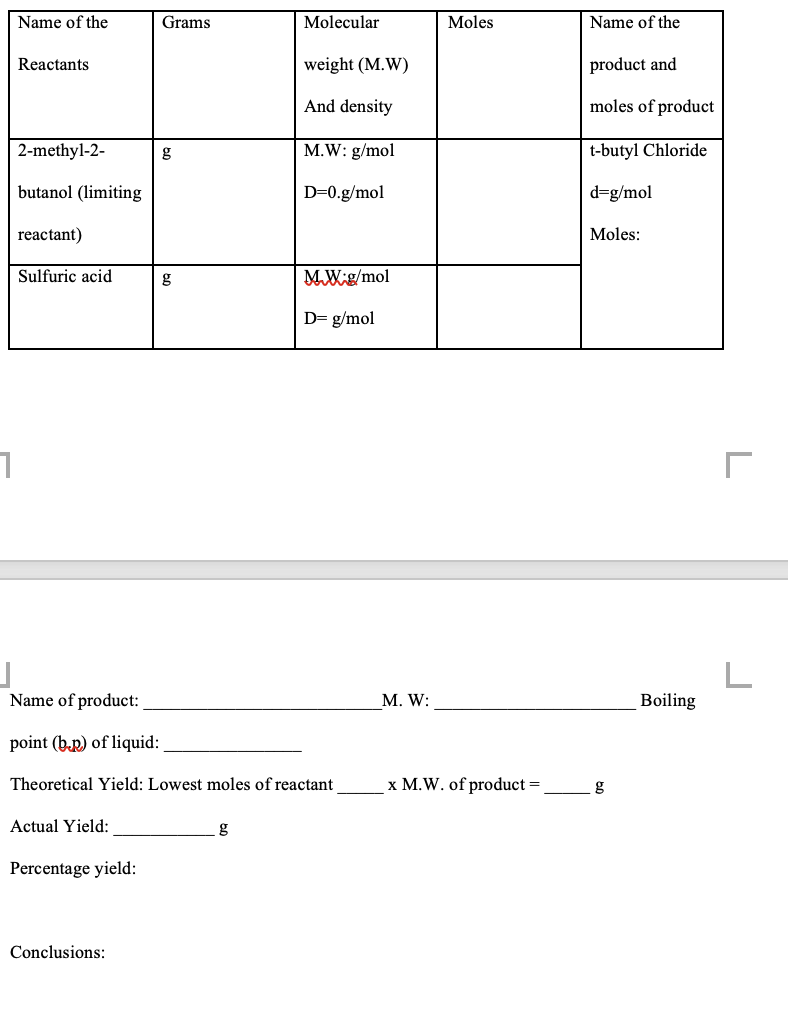
Please write a conclusion too Actual yield of 2-methyl-2-butene is 3.0 g. To calculate grams use for the limiting reactant 2-methyl-2-butanol, 5.0 mL, 4.0 g,
Please write a conclusion too
Actual yield of 2-methyl-2-butene is 3.0 g. To calculate grams use for the limiting reactant 2-methyl-2-butanol, 5.0 mL, 4.0 g, use d =M / V (density = 0.809 g/mL). 85% phosphoric acid: 85% means in 100 mL it is 85 g, in 10.0 mL = 8.5 g, 0.087 mol, m.wt. 98 g/mol [85% phosphoric acid (15.2 M), d = 1.68 g/mL]. Use phosphoric acid in the table because it is environmentally friendly. We can also use sulfuric acid that is not environmentally friendly.
In addition, make sure in the lab Report you write the following.
In addition, make sure in the lab Report you write the following: 1. Balanced chemical equation. 2. Mechanism using the arrows
Name of the Grams Molecular Moles Name of the Reactants weight (M.W) product and And density moles of product 2-methyl-2- 8 M.W: g/mol t-butyl Chloride butanol (limiting D=0.g/mol d=g/mol reactant) Moles: Sulfuric acid g MW g/mol D=g/mol 1 j Name of product: L M. W: Boiling point (b.p) of liquid: Theoretical Yield: Lowest moles of reactant x M.W. of product = g Actual Yield: Percentage yield: ConclusionsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


