Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please write in clear hand writing References Use the References to access important values if needed for this question. 900 Vapor pressure (mm Hg) 800
please write in clear hand writing 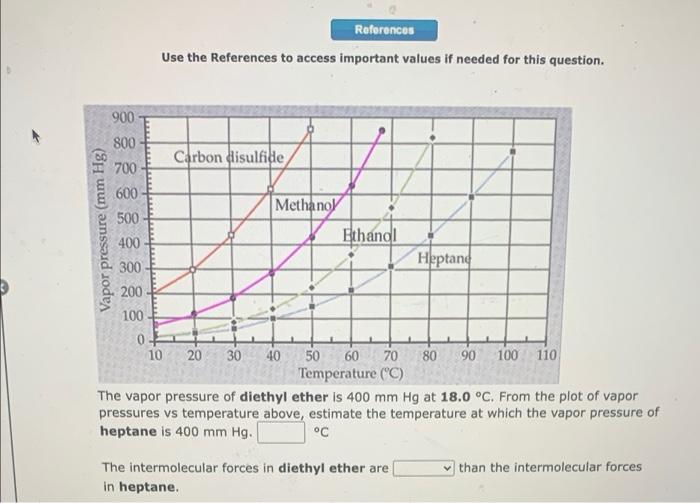
References Use the References to access important values if needed for this question. 900 Vapor pressure (mm Hg) 800 Carbon disulfide 700 600 Methanol 500 Ethanol 400 300 Heptane 200 100 0- 10 20 30 40 50 60 70 80 90 100 110 Temperature (C) The vapor pressure of diethyl ether is 400 mm Hg at 18.0 C. From the plot of vapor pressures vs temperature above, estimate the temperature at which the vapor pressure of heptane is 400 mm Hg. C than the intermolecular forces The intermolecular forces in diethyl ether are in heptane 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


