Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plesse help me in answer these five questions and there is uploded of the report sheet as it nedded to answer the question: POST-LAB QUESTIONS
plesse help me in answer these five questions and there is uploded of the report sheet as it nedded to answer the question: 
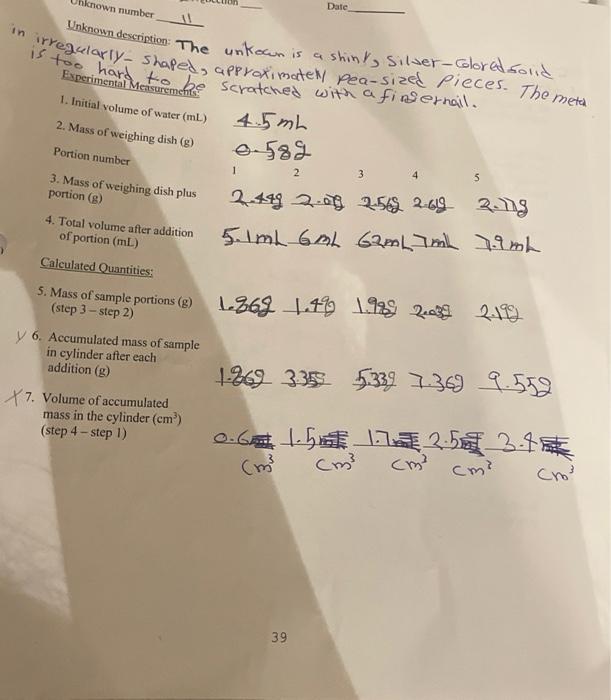

POST-LAB QUESTIONS 1. Estimate the uncertainty in your individual mass and volume measurements by computing the relative precision of one mass and one volume measurement. Use the mass and volume of portion 1 of your unknown only. Be sure to show the equation used before inserting the numbers. Which is greater, the uncertainty in the mass measurement or that in the volume measurement? 2. If an air bubble adhered to the surface of the metal when submerged in the water, would the experimentally determined density be too high, too low, or unaffected? Briefly explain your answer. Hint: for portion 1 of your unknown, assume that the volume of water being displaced by the air bubble is 0.2 cm3, then recompute the density of the portion (as in step 13 of report sheet 2). 3. What must be changed in the calculation of the 95% confidence limits to obtain a different degree of confidence, for example 99%. 4. Why will a 99% Confidence Interval always be wider than a 95% Confidence Interval for a given sample of observations? 5. Since the volume of zero mass should be zero, the Y-intercept of the regression line should also, ideally, be zero. Give a reasonable explanation for why your experimental value of the Y-intercept (step 9 of report sheet 1) was not equal to zero. known number Date Unknown description: The unkon is a shiny, Silver-colored.solid in irregularly- Shaped, approximate Pea-size pieces. The metal is too hard to be scratched with a fingernail. 4.5mL 0.589 Experimental Measurements 1. Initial volume of water (ml) 2. Mass of weighing dish (8) Portion number 1 2 3 4 5 3. Mass of weighing dish plus portion () 4. Total volume after addition of portion (mL) Calculated Quantities: 2.49 2.03 2548 2019 2ung 5. mL GAL 62mLmL 7.9mL 5. Mass of sample portions (e) 1363 1.4 1.1 2.38 219 (step - ) y 6. Accumulated mass of sample in cylinder after each addition () 1869 335 5339 7369 9.552 77. Volume of accumulated mass in the cylinder (cm) (step 4 - step 1) o.Gethin 11225 3.4 cm? cm cm? cm? (m? 39 dure Linear Regression 8. Slope of the line 2.8537 9. Intercept of the line -0.04.63 10. Density of the solid (w/cm) 2.85 dicm 11. Volumes used to compute the corresponding mass on the line (ml) x 1.5 12. Corresponding mass on the line (8) Y: 5.5 Procedure 2: Mean Value and Confidence Interval for the True Mean x 2 Y, 4 Portion number 1 2 3 4 5 280 g/cm n - dicm 13. Individual density measures (g/cm) [Step 6/Step 71 3.1g. lcm 2.23 3.13 294 21cm dlom alom Mean Density (Sem) 2.24 Standard deviation (9cm) 2.2123x10 g/m Relative variability (%) 11.50 - 5df-4 4(095, n-1)-2-776 Standard error of the mean (g/em') 1.463Xlo Uncertainty of the mean (g/cm) 0.406 95% confidence limits (g/cm) [2-43453.240] Calculation of the standard error, uncertainty, 95% confidence limits for the true mean (show the equations used before inserting the values): * Standard error=s = 0.32723 = 0.146 =11.463 xlo uncertainty = fx sluna 15 2.776 x ) = 0.706 =1.06X16 upper limit=2.84 +0.406 hower limit=2.84 -0.406 Calculation of Y, from X,: 9500 Confidence limits for Identity of element (name and symbol) = 4.23-0 A Calcm m2 Aluminum (A) 40 (2-4347 3.246) $95% confidence : Y I txsiva 7 bo+bx=-6-0463+ (2-853) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


