Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Plot a polarization curve [current density (Amperes/cm2 ) versus potential (Volt)] for the following electrode reaction between 230 mV and 230 mV. Assume that both
Plot a polarization curve [current density (Amperes/cm2 ) versus potential (Volt)] for the following electrode reaction between 230 mV and 230 mV.
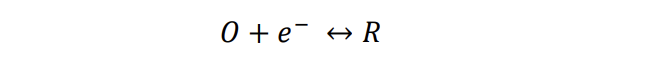 Assume that both ohmic and concentration polarizations are absent: *= 1.50 , * = 0.50 , 0' = 0.0500 , 0 = 4.0106 /, = 0.60, = 280 . You can use a spreadsheet, Matlab, Octave, Scilab, etc. Show your solution method (computercode, excel formula, etc.) as well. As a calculation example, calculate by hand the value of current density at = -0.1500 Volt.
Assume that both ohmic and concentration polarizations are absent: *= 1.50 , * = 0.50 , 0' = 0.0500 , 0 = 4.0106 /, = 0.60, = 280 . You can use a spreadsheet, Matlab, Octave, Scilab, etc. Show your solution method (computercode, excel formula, etc.) as well. As a calculation example, calculate by hand the value of current density at = -0.1500 Volt.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


