Answered step by step
Verified Expert Solution
Question
1 Approved Answer
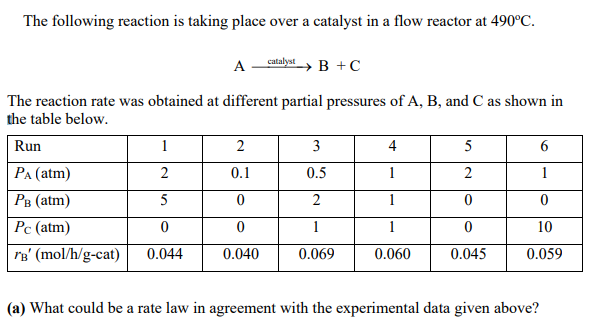
PLOT USING POLYMATH The following reaction is taking place over a catalyst in a flow reactor at 490C. AcatalystB+C The reaction rate was obtained at
PLOT USING POLYMATH
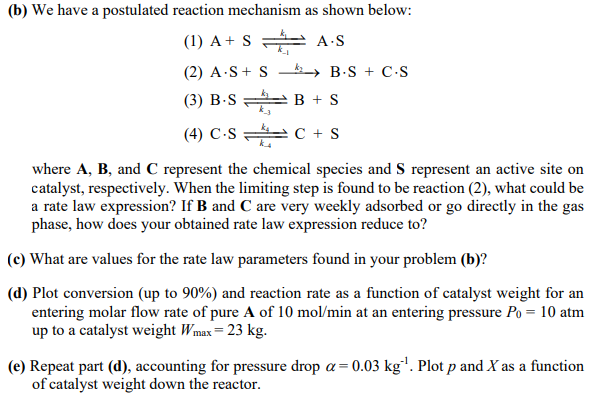
The following reaction is taking place over a catalyst in a flow reactor at 490C. AcatalystB+C The reaction rate was obtained at different partial pressures of A,B, and C as shown in the table below. (a) What could be a rate law in agreement with the experimental data given above? (b) We have a postulated reaction mechanism as shown below: (1) A+Sk1k1AS (2) AS+Sk2BS+CS (3) BSk3k3B+S (4) CSk4k4C+S where A,B, and C represent the chemical species and S represent an active site on catalyst, respectively. When the limiting step is found to be reaction (2), what could be a rate law expression? If B and C are very weekly adsorbed or go directly in the gas phase, how does your obtained rate law expression reduce to? (c) What are values for the rate law parameters found in your problem (b)? (d) Plot conversion (up to 90% ) and reaction rate as a function of catalyst weight for an entering molar flow rate of pure A of 10mol/min at an entering pressure P0=10atm up to a catalyst weight Wmax=23kg. (e) Repeat part (d), accounting for pressure drop =0.03kg1. Plot p and X as a function of catalyst weight down the reactor
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


