Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pls answer all parts, thank you a. (i) In the HPLC analysis of a mixture of compounds A and B, the more efficient the column
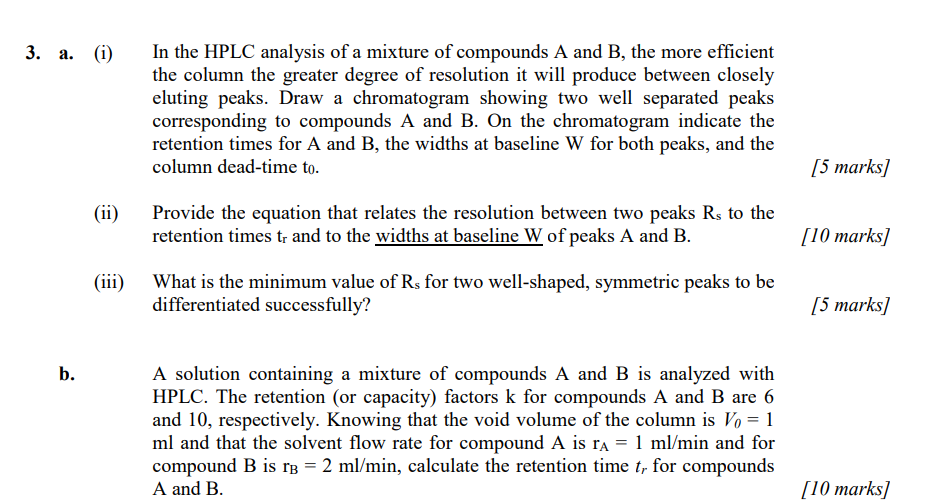
Pls answer all parts, thank you
a. (i) In the HPLC analysis of a mixture of compounds A and B, the more efficient the column the greater degree of resolution it will produce between closely eluting peaks. Draw a chromatogram showing two well separated peaks corresponding to compounds A and B. On the chromatogram indicate the retention times for A and B, the widths at baseline W for both peaks, and the column dead-time t0. (ii) Provide the equation that relates the resolution between two peaks Rs to the retention times tr and to the widths at baseline W of peaks A and B. (iii) What is the minimum value of Rs for two well-shaped, symmetric peaks to be differentiated successfully? b. A solution containing a mixture of compounds A and B is analyzed with HPLC. The retention (or capacity) factors k for compounds A and B are 6 and 10 , respectively. Knowing that the void volume of the column is V0=1 ml and that the solvent flow rate for compound A is rA=1ml/min and for compound B is rB=2ml/min, calculate the retention time tr for compounds A and BStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


