Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pls help me with the questions below. Thank you! NH4Cl Used in Fertilizer Production Dissociation of Salt: hydrolysix: Value of K _ of NH= After
Pls help me with the questions below. Thank you! 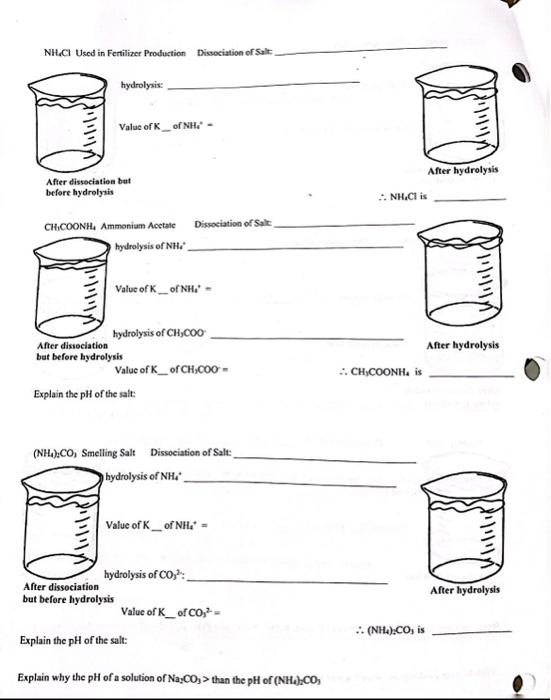
NH4Cl Used in Fertilizer Production Dissociation of Salt: hydrolysix: Value of K _ of NH= After hydrolysis After disseciatiea but before hydrolysis NH4Cl is CH1COONH4 Ammonium Acctate Dissociation of Salte hydrolysis of NH Value of \( \mathrm{K}_{\text {_ }} \) of NH= hydrolysis of CHOCOO After disiociation After hydrolysis bat before hydrolysis Value of K_of CH3COO= CHyCOONH4 is is Explain the pH of the salt: (NH4)2CO3 Smelling Salt Dissociation of Salt: | hydrolysis of NH4 * Value of K _ of NH4= 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


