Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plz help in all An intermediate step in the industrial production of nitric acid involves the reaction of ammonia with oxygen gas to form nitrogen
plz help in all 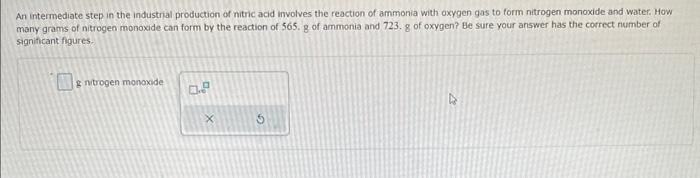
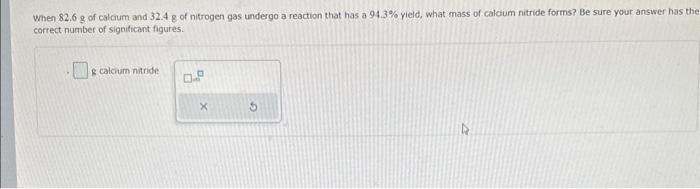
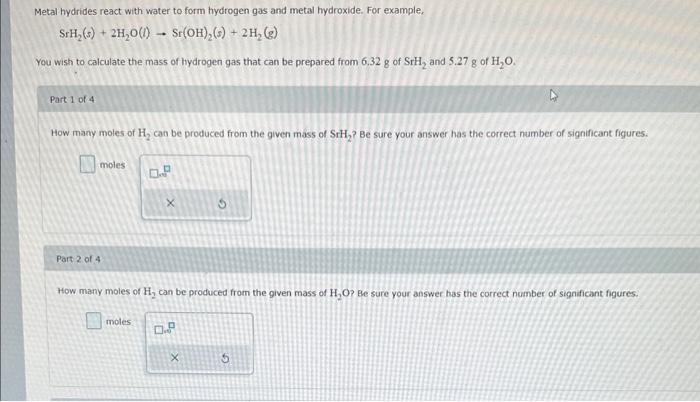
An intermediate step in the industrial production of nitric acid involves the reaction of ammonia with oxygen gas to form nitrogen monoxide and water. Mow many grams of nitrogen monoxide can form by the reaction of 565 . g of ammonia and 723.8 of oxygen? Be sure your answer has the correct number of significant figures. S nitrogen monoxide When 82.6g of calcum and 32.48 of nitrogen gas undergo a reaction that has a 94.3% yield, what mass of calcum nitride forms? Be sure your answer has the correct number of significant figures. e calcium nitride SrH2(s)+2H2O(t)Sr(OH)2(s)+2H2(g) You wish to calculate the mass of hydrogen gas that can be prepared from 6.32g of SrH2 and 5.27g of H2O. Part 1 of 4 How many moles of H2 can be produced from the given mass of SrH2 ? Be sure your answer has the correct number of significant figures. moles Part 2 of 4 How many moles of H2 can be produced from the given mass of H2O ? Be sure your answer has the correct number of significant figures. moles 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


