Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Plz quick i need just the answer From the table below for distribution of solute between two immiscible solvent If 20 ml of lodine/water was
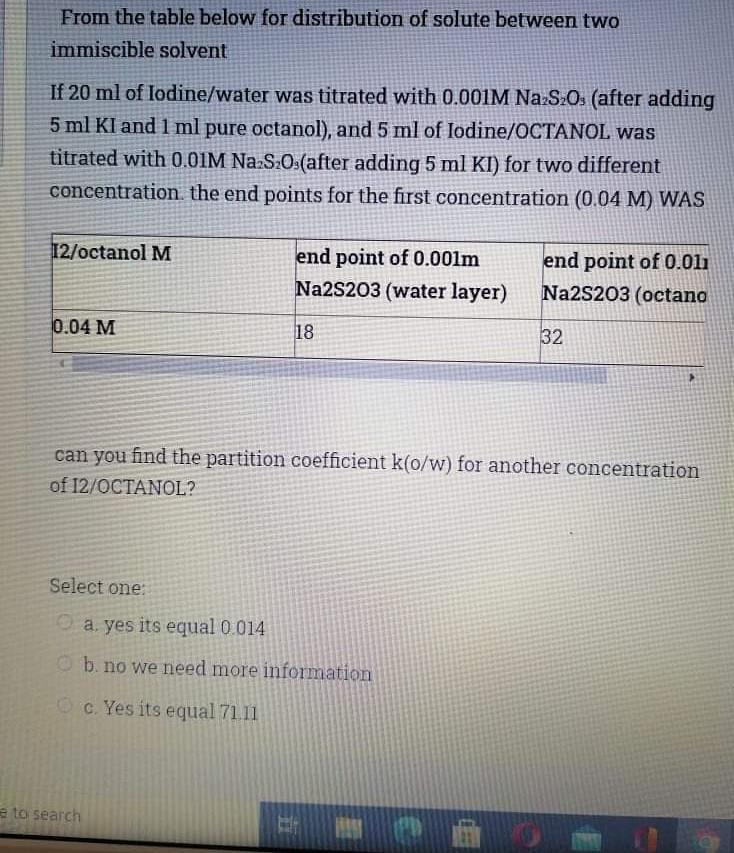
Plz quick i need just the answer
From the table below for distribution of solute between two immiscible solvent If 20 ml of lodine/water was titrated with 0.001M Na S20s (after adding 5 ml KI and 1 ml pure octanol), and 5 ml of lodine/OCTANOL was titrated with 0.01M Na2S2O (after adding 5 ml KI) for two different concentration the end points for the first concentration (0.04 M) WAS 12/octanol M end point of 0.001m Na2S203 (water layer) end point of 0.011 Na2S203 (octano 0.04 M 18 32 can you find the partition coefficient k(o/w) for another concentration of 12/OCTANOL? Select one a. yes its equal 0.014 b. no we need more information c. Yes its equal 71.11 to searchStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


